Titanium Atom That Exists in Two Places at Once in Crystal to Blame for Unusual Phenomenon
Researchers discover why a perfect crystal is not good at conducting heat, although it seemingly should be
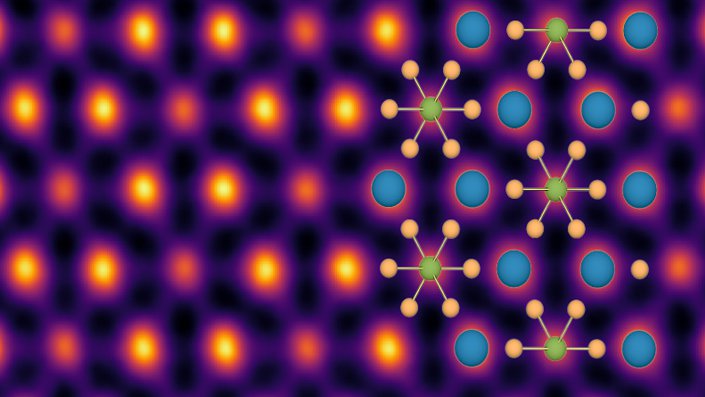
The crystalline solid BaTiS3 (barium titanium sulfide) is terrible at conducting heat, and it turns out that a wayward titanium atom that exists in two places at the same time is to blame.
The discovery, made by researchers from Caltech, USC, and the Department of Energy’s Oak Ridge National Laboratory (ORNL), was published on November 27 in the journal Nature Communications. It provides a fundamental atomic-level insight into an unusual thermal property that has been observed in several materials. The work is of particular interest to researchers who are exploring the potential use of crystalline solids with poor thermal conductivity in thermoelectric applications, in which heat is directly converted into electric energy and vice versa.
“We have found that quantum mechanical effects can play a huge role in setting the thermal transport properties of materials even under familiar conditions like room temperature,” says Austin Minnich, professor of mechanical engineering and applied physics at Caltech and co-corresponding author of the Nature Communications paper.
Crystals are usually good at conducting heat. By definition, their atomic structure is highly organized, which allows atomic vibrations—heat—to flow through them as a wave. Glasses, on the other hand, are terrible at conducting heat. Their internal structure is disordered and random, which means that vibrations instead hop from atom to atom as they pass through.
BaTiS3 belongs to a class of materials called Perovskite-related chalcogenides. Jayakanth Ravichandran, an assistant professor in USC Viterbi’s Mork Family Department of Chemical Engineering and Materials Science, and his team have been investigating them for their optical properties and recently started studying their thermoelectric applications.
“We had a hunch that BaTiS3 will have low thermal conductivity, but the value was unexpectedly low. Our study shows a new mechanism to achieve low thermal conductivity, so the next question is whether the electrons in the system flow seamlessly unlike heat to achieve good thermoelectric properties,” says Ravichandran.
The team discovered that BaTiS3, along with several other crystalline solids, possessed “glass-like” thermal conductivity. Not only is its thermal conductivity comparable to those of disordered glasses, it actually gets worse as temperature goes down, which is the opposite of most materials. In fact, its thermal conductivity at cryogenic temperatures is among the worst ever observed in any fully dense (nonporous) solid.
The team found that the titanium atom in each BaTiS3 crystal exists in what is known as a double-well potential—that is, there are two spatial locations in the atomic structure where the atom wants to be. The titanium atom existing in two places at the same time gives rise to what is known as a “two-level system.” In this case, the titanium atom has two states: a ground state and an excited state. Passing atomic vibrations are absorbed by the titanium atom, which goes from the ground to the excited state, then quickly decays back to ground state. The absorbed energy is emitted in the form of a vibration and in a random direction.
The overall effect of this absorption and emission of vibrations is that energy is scattered rather than cleanly transferred. An analogy would be shining a light through a frosted glass, with the titanium atoms as the frost; incoming waves deflect off of the titanium, and only a portion make their way through the material.
Two-level systems have long been known to exist, but this is the first direct observation of one that was sufficient to disrupt thermal conduction in a single crystal material over an extended temperature range, measured here between 50 and 500 Kelvin.
The researchers observed the effect by bombarding BaTiS3 crystals with neutrons in a process known as inelastic scattering using the Spallation Neutron Source at ORNL. When they pass through the crystals, the neutrons gain or lose energy. This indicates that energy is absorbed from a two-level system in some cases and imparted to them in others.
“It took real detective work to solve this mystery about the structure and dynamics of the titanium atoms. At first it seemed that the atoms were just positionally disordered, but the shallowness of the potential well meant that they couldn’t stay in their positions for very long,” says Michael Manley, senior researcher at ORNL and co-corresponding author of the Nature Communications paper. That’s when Raphael Hermann, researcher at ORNL, suggested doing quantum calculations for the double well. “That atoms can tunnel is well known, of course, but we did not expect to see it at such a high frequency with such a large atom in a crystal. But the quantum mechanics is clear: if the barrier between the wells is small enough, then such high-frequency tunneling is indeed possible and should result in strong phonon scattering and thus glass-like thermal conductivity,” Manley says.
The conventional approach to creating crystalline solids with low thermal conductivity is to create a lot of defects in those solids, which is detrimental to other properties such as electrical conductivity. So, a method to design low-thermal-conductivity crystalline materials without any detriment to electrical and optical properties is highly desirable for thermoelectric applications. A small handful of crystalline solids exhibit the same poor thermal conductivity, so the team next plans to explore whether this phenomenon is to blame in those materials as well.
The Nature Communications article is titled “High frequency atomic tunneling yields ultralow and glass-like thermal conductivity in chalcogenide single crystals.” Co-authors include Bo Sun, Jaeyun Moon, and Nina Shulumba of Caltech; Shanyuan Niu, Boyang Zhao, JoAnna Milam-Guerrero, Ralf Haiges, Brent C. Melot, and Matthew Mecklenburg of USC; Raphael P. Hermann, Katharine Page, and Barry Winn of Oak Ridge National Laboratory; Arashdeep S. Thind and Rohan Mishra of Washington University in St. Louis; Krishnamurthy Mahalingam and Brandon M. Howe of the Air Force Research Laboratory at Wright-Patterson Air Force Base; Young-Dahl Jho of Gwangju Institute of Science and Technology in South Korea; and Ahmet Alatas of Argonne National Laboratory in Illinois.
This research was supported by the Defense Advanced Research Projects Agency, the U.S. Department of Energy, the Office of Naval Research, the National Science Foundation, the Army Research Office, the Air Force Office of Scientific Research and the Link Foundation.