Zhejiang University scientists unveil major roles of hexokinase 2 in shaping microglial functions
On December 20, the research team led by Prof. GAO Zhihua and Prof. DUAN Shumin at the Zhejiang University School of Brain Science and Brian Medicine published an open-access research article titled “Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity”.
Microglia, the primary immune cells and key guardians of brain activity in the brain, can constantly monitor the microenvironment to maintain brain homeostasis. When subjected to acute depletion or under disease conditions, they will undergo rapid proliferation, resulting in restoration of the homeostatic poor or formation of microglial clusters surrounding disease sites. In addition, microglia quickly migrate towards the damage sites and elicit immune responses to restrain and repair the damage. However, little is known about the specific molecular determinants that metabolically and bioenergetically shape microglial functions.
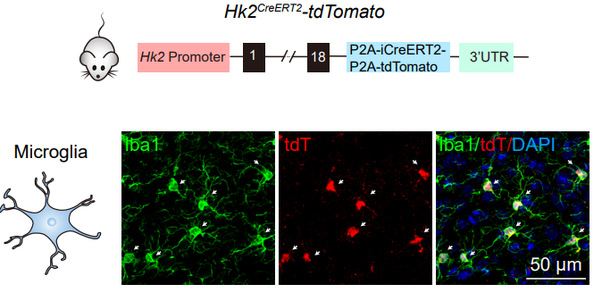
Hexokinase (HK), by catalyzing phosphorylation of glucose to glucose-6-phosphate, is the first rate-limiting enzyme in glucose utilization. Four main HK isozymes, HK1, HK2, HK3 and HK4, with different biochemical features and catalytic activities have been identified. By systematically analyzing the metabolic regulator in different brain cell types, Dr. HU Yaling et al. unexpectedly found that HK2, the most active isozyme associated with mitochondrial membrane, is selectively expressed in microglia in the brain.
In the study, the researchers discovered that genetic ablation of HK2 reduced microglial glycolytic flux and energy production, suppressed microglial repopulation, and attenuated microglial surveillance and damage-triggered migration in male mice. HK2 elevation was particularly prominent in immune-challenged or disease-associated microglia. In ischaemic stroke models, however, HK2 deletion promoted neuroinflammation and potentiated cerebral damages. The enhanced inflammatory responses after HK2 ablation in microglia were thus found to be associated with aberrant mitochondrial function and reactive oxygen species accumulation.
This study demonstrates that HK2 gates both glycolytic flux and mitochondrial activity to shape microglial functions, which contribute to metabolic abnormalities and maladaptive inflammation in brain diseases. This will open the door to potential therapeutic targeting of microglia in multiple neurological diseases in which HK2 elevation and metabolic abnormalities are implicated.