University Of Southampton’s Clinical Trial Gets First Patients To Improve Heart Failure Treatment
The first patient has undergone surgery in a landmark clinical trial which aims to improve heart failure treatment.
Heart patient Phil O’Donoghue is taking part in the BRITISH study, which is funded by £1.8m from the British Heart Foundation to work out which patients with heart failure may benefit from having a defibrillator fitted under the skin in their chest to shock the heart if it goes into cardiac arrest.
The study is being led by Dr Andrew Flett, consultant cardiologist at University Hospital Southampton (UHS), and Professor Nick Curzen, Professor of Interventional Cardiology at the University of Southampton, and is co-ordinated by the NIHR Southampton Clinical Trials Unit.
Over 2,500 patients due to be recruited across the UK in the next three years.
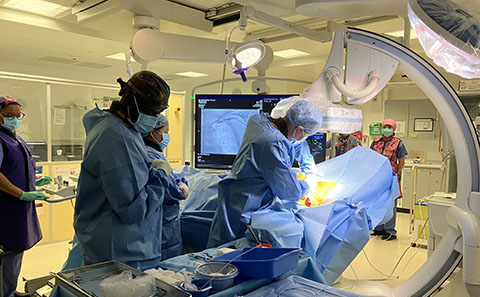
First patient
Phil O’Donoghue, 53, suffers from non-ischemic cardiomyopathy (NICM). This is a common type of heart failure which can lead to abnormal heart rhythms, and sudden cardiac arrests are a possible cause of death in these patients.
Phil, who is from Chandler’s Ford in Hampshire, went to accident and emergency during the first Covid-19 lockdown in May 2020 after suffering palpitations and feeling unable to breath and was initially put on medication. He says the diagnosis of heart failure has had a big effect on his day-to-day life.
“I can’t do the things I want to do if it means exerting myself. I had to change what I did at physically at work. I do a lot of fishing for relaxation but pushing all my gear on the cart means I get very breathless. I used to ride motorbikes and do track days but pushing and pulling the bike round is hard work.”
Phil was on the medication for two and a half years but then suffered further episodes in January this year and then again in March.
“I was rushed into hospital and tests showed my heart’s ejection rate was down to 24% which meant blood was not being pumped around my body properly. I was told there was a trial about to start and that I fitted what they were looking for.”
“I wanted to do the trial,” says Phil. “My mum was a nurse for forty years. When my daughter was born, doctors followed her for 16 years looking at things like bone density. So, I’ve always been involved in research, my mum’s always been involved in it. When I spoke to Dr Flett it wasn’t even a question of shall I do it or shall I not, it made perfect sense to go ahead and do the trial.”
Assessing which patients will benefit
Heart failure effects over 900,000 people in the UK, with around 200,000 new cases diagnosed each year*, and an estimated cost of £2bn to the NHS.
Implantable cardioverter defibrillators (ICDs) are small devices that are routinely fitted in the chests of patients with heart failure. They can stop abnormal rhythms and treat cardiac arrest by delivering an electric shock to the heart.
“The current guidelines look at how well the heart is pumping to decide which patients should get a defibrillator,” says Dr Andrew Flett, Chief Investigator of the BRITISH trial. “But for many patients who go through the procedure of having a defibrillator fitted, they will never actually see the device triggered and may not need it. We therefore want to find a better way to assess which patients will truly benefit from one of these devices.
“There is evidence that scar tissue in the heart muscle may be the cause of dangerous heart rhythms for patients with NICM. This will be the first ever trial to look at whether the presence of scar tissue can predict who should be fitted with an ICD.”
An MRI scan is used to detect the presence of scar tissue in the heart and those patients are invited into the trial.
“Participants are randomly allocated to one of two trial arms,” says Professor Nick Curzen, co-investigator on the trial. “Half will be fitted with an implantable defibrillator. The others will be fitted with an implantable loop recorder (ILR), a device which monitors heart activity so that the team can review any abnormal rhythms, but which does not shock the heart.”
Phil is in the defibrillator arm and this week was the first patient enrolled in the trial to have the device fitted at University Hospital Southampton.
“Having the defibrillator doesn’t bother me, it’s just one of those things. If it goes off, it goes off. And if it doesn’t, then great. But if the trial can show one way or another whether they should be used, and move treatments forward, then it’s obviously a benefit.”
Hitesh Mistry, from Southampton, is also taking part the trial.
The 48-year-old didn’t know he had a heart condition until last summer when a terrifying episode left him unable to breathe.
“I had been treated for anaemia for several years after generally feeling run-down, tired and unwell. But then one night during the hot weather last summer I tried to go to bed and when I lay down, I couldn’t breathe. Each time I lay down, I just couldn’t breathe.”
Hitesh was taken for an x-ray the following day which found fluid on his lungs that was restricting his breathing, and following further tests went straight in to see a cardiologist.
“I was told that my heart’s not working properly, and the doctors needed to give me some medication otherwise I’d end up in hospital and potentially very unwell. I was pretty shocked as I had no idea that I had a heart condition. The doctors also don’t know how long I’ve had it for but believe it was causing the anaemia, so it may have been some time.”
Hitesh is on the second arm and will be having a loop recorder fitted.
“I feel reassured that rather than just relying on the pills, something more is going happen. The activity of my heart is being recorded and goes back to the hospital to be monitored.
“But one of the reasons I agreed to be part of the trial is that it’s looking at something that’s unknown at the moment and will hopefully help other people like me in the future by. I’m all for helping out with research.”
Improving treatment
The recruitment of the first patients to the BRITISH trial comes during the British Heart Foundation’s ‘The This is Science’ campaign, raising awareness of the lifesaving research the charity funds.
X-ray of ICD implant in patient’s chest
X-ray of ICD implant in patient’s chest
Professor Sir Nilesh Samani, Medical Director of the British Heart Foundation, said: “ICDs are crucial devices to treat sudden cardiac arrest and save lives. But it is important that we continue to establish exactly which patients need them, so that people who are unlikely to benefit do not have to undergo invasive procedures unnecessarily.
“The BRITISH trial will test whether the presence of scar tissue in the heart predicts who will benefit most from having an ICD. Funding the trial demonstrates the BHF’s continuing commitment to improving the treatment of patients with heart disease, by gathering the best evidence through high-quality research.”
The trial is being conducted by the Southampton Clinical Trials Unit (SCTU) who are responsible for running the study at participating hospitals across the UK.
“The trial will also involve a registry of patients who do not have scar tissue and are therefore treated according to current guidelines,” says Anna Cebula, Trial Manager for BRITISH at the SCTU. “This means we will be able to review data from all three groups and compare patient outcomes to establish whether scar tissue is an indicator of who should be fitted with an ICD”.
The trial is currently open to patients at five hospitals in Southampton, Portsmouth, Aberdeen, East Kent, and Barts in London, with a further 30 sites to open in the coming months.
Dr Flett concludes, “The ultimate goal of any research is to improve treatment for our patients. The BRITISH trial will inform UK and international guidelines for the treatment of heart failure. Depending on the outcome of the study, it could identify a group of patients who we know will benefit from ICDs and reduce unnecessary procedures for patients who don’t need them ensuring cost effective use of our health service in the future.”