Thin-Film Neural Electrodes: A Revolution in Brain Monitoring and Stimulation
Flexible thin-film electrodes placed directly on brain tissue show promise for the diagnosis and treatment of epilepsy, as demonstrated recently by scientists at Tokyo Tech. Thanks to an innovative yet straightforward design, these durable electrodes accurately match the mechanical properties of brain tissue, leading to better performance during electrocorticography recordings and targeted neural stimulation.
Measuring brain activity is a useful technique for diagnosing epilepsy and other neuropsychiatric disorders. Among the several approaches adopted, electroencephalography (EEG) is the least invasive. During EEG recordings, electrodes are typically placed on the scalp. However, this limits the resolution of EEG as the electrical signals from the brain are attenuated and distorted by the time they reach the scalp.
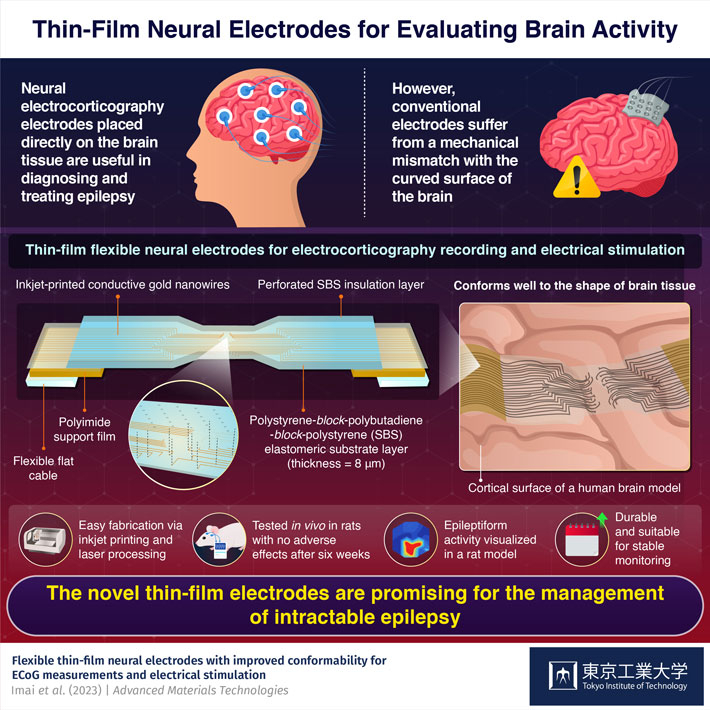
In contrast, electrocorticography (ECoG) involves placement of neural electrodes directly on the surface of the brain. Being in close contact with the region of interest, ECoG electrodes provide better recordings of brain activity. Moreover, it is also possible to send electrical pulses through them to stimulate specific groups of neurons with the aim of managing epileptic seizures. However, conventional ECoG electrodes have a major drawback. They usually do not match the mechanical properties and curvature of brain tissue, resulting in increased brain pressure and other adverse effects. Although soft neural electrodes have been developed to mitigate this issue, they either lack durability and strength or require complex fabrication processes.
To address these problems, a research team guided by Associate Professor Toshinori Fujie of Tokyo Institute of Technology (Tokyo Tech) has developed a new type of flexible neural electrode. Their design and findings, recently published in Advanced Materials Technologies, can revolutionize how ECoG recordings and direct neural stimulation are performed.
The substrate of the proposed electrode consists of a thin film made of a flexible material called polystyrene-block-polybutadiene-block-polystyrene (SBS). The researchers used an inkjet printer to fabricate conductive wiring on the electrode with gold nanoink. Finally, they covered the circuit by stacking another SBS layer as insulation, with laser-perforated microchannels as measurement or stimulation points.
Through extensive mechanical testing and simulations, the researchers demonstrated that the electrode accurately conforms to the shape of brain tissue containing many irregular ridges. Its straightforward design and fabrication process is a major advantage as well, since it is conducive to the widespread adoption of the proposed electrode in practical applications. “As far as we know, this is the first study to demonstrate such ultra-conformable ECoG electrodes based on printed electronics, which closely match the mechanical properties of brain tissue,” highlights Dr. Fujie.
To showcase the potential of their design, the team conducted several experiments on epilepsy rat models. Using the newly designed ECoG electrodes, they could accurately measure the neural response in the brains of these rats when one of their whiskers was mechanically stimulated. Additionally, they could visualize seizure activity during a chemically-induced epilepsy. Further, by triggering movement in the rats’ whiskers and arms via electric pulses sent through specific channels, the researchers demonstrated that the proposed electrodes can stimulate different regions of the brain.
Overall, these findings highlight the potential of flexible thin-film neural electrodes for the diagnosis and treatment of epilepsy and other brain diseases. Notably, the electrodes did not cause any inflammation or adverse effects in the rats’ brains even several weeks after the procedure, highlighting their compatibility with biological tissue.
The researchers plan on improving their design further to make it suitable for clinical applications. “The integration of our thin-film electrode with an implantable device could make it even less invasive and more sensitive to the brain’s abnormal electrical activity,” explains Dr. Fujie. “This would enable improved diagnostics and therapeutic strategies for the management of intractable epilepsy.”

