Elastic Deformation’s Role in Sodium-Ion Battery Cathode Charging Unveiled by Microstructure Simulations
Research into new battery materials is not only aimed at optimizing performance and service life and reducing costs. Rather, it is also about reducing rare elements such as lithium and cobalt as well as toxic components. Sodium-ion batteries, which are based on similar principles to lithium-ion batteries but can be produced from raw materials that are sufficiently available in Europe, are considered promising. They are suitable for stationary and mobile applications. “Layered oxides such as sodium-nickel-manganese oxides are promising as materials for the cathode,” reports Dr. Simon Daubner, group leader at the Institute for Applied Materials – Microstructure Modeling and Simulation (IAM-MMS) at KIT and corresponding author of the study. In the Cluster of Excellence POLiS (stands for: Post Lithium Storage ) he researches sodium ion technology.
Mechanical stress occurs during fast charging
However, there is a problem with these cathode materials: sodium-nickel-manganese oxides change their crystal structure depending on how much sodium is currently stored. If the material is loaded slowly, everything goes smoothly. “The sodium comes out of the material layer by layer – like in a parking garage that is emptying floor by floor,” explains Daubner. “But if things have to happen quickly, the sodium is pulled out from all sides.” This leads to mechanical stresses that can permanently damage the material.
Researchers at the Institute for Nanotechnology (INT) and at the IAM-MMS at KIT, together with scientists at the University of Ulm and at the Center for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW), have now uncovered these connections using simulations and are reporting about it in npj Computational Materials, a journal from the Nature portfolio.
Experiments confirm simulation results
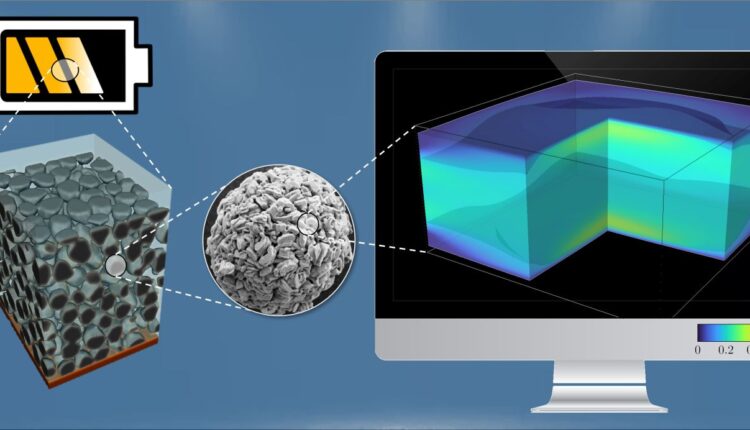
“Computer models can describe different length scales, from the arrangement of atoms in electrode materials to their microstructure to the cell as a functional unit of every battery,” says Daubner. These combine microstructure models with slow charge and discharge experiments to investigate the layered oxide NaXNi1/3Mn2/3O2. The material has several degradation mechanisms that lead to loss of capacity. Therefore, it is currently not suitable for commercial applications. When the crystal structure changes, elastic deformation occurs. The crystal shrinks, which can cause cracks and reduce available capacity. As simulations carried out at INT and IAM-MMS showed, this mechanical influence is so strong that it significantly influences how quickly the material can be loaded. Experimental studies at ZSW confirmed the results.
The findings gained in the study can partly be transferred to other layered oxides. “Now that we understand the basic processes, we can devote further work to developing battery materials that are long-lasting and can be charged as quickly as possible,” summarizes Daubner. This could make the large-scale use of sodium-ion batteries a reality in five to ten years.