USTC Unveils New Link Between Metal Loading and Acidic Oxygen Evolution
According to a study published in ACS Catalysis, a research team led by Prof. YAN Wensheng from the University of Science and Technology of China (USTC) has revealed the “volcano-type” relationship between metal loading and acidic oxygen evolution reaction (OER) activity in single-atom catalysts.
Addressing the challenge of fabricating atomic-level dispersed metal catalysts with elevated metal loadings represents a formidable hurdle in the single-atom catalysis. Moreover, elucidating the intricate interplay between the microscopic electronic interactions among metals at high loadings and their influence on the macroscopic catalytic behavior holds great importance for refining and optimizing the metal loading strategy.
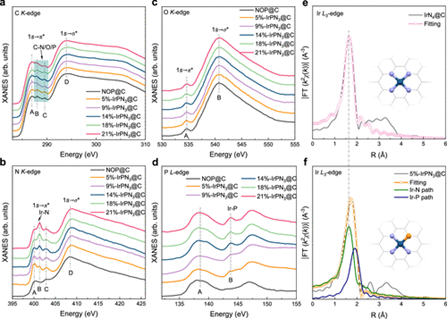
The researchers adopted a simple P-anchoring strategy and successfully synthesized a series of Ir single-atom catalysts (x-IrPN3@C) with metal loadings ranging from 5% to 21wt%. Through synchrotron radiation XAS spectroscopy, the team clarified that the existence of the Ir-P coordination structure was the intrinsic reason for achieving high Ir atom loading.
This coordination structure stabilized the Ir atoms and prevented their aggregation at high loadings. Catalytic performance tests showed that the relationship between the metal loading of this catalyst and the catalytic activity of the acidic oxygen evolution reaction (OER) did not exhibit a simple “positive correlation”, but rather a unique “volcano-type” relationship.
The research team clarified the underlying mechanism of the “volcano-type” relationship with the help of XAS (X-ray Absorption Spectra), X-ray photoelectron spectroscopy (XPS), and theoretical calculations. When the Ir loading went up within a certain range, the number of active sites increased, thereby enhancing the OER catalytic activity. Yet when loading exceeded a certain threshold, the interaction between adjacent Ir atoms intensified, leading to a decrease in the valence state of Ir and subsequently reducing the intrinsic OER activity.
The competition between the increase in the number of active sites and the decrease in their intrinsic activity at high densities contributed to the “volcano-type” curve.
This research provides theoretical guidance for designing more efficient and economical single-atom catalysts for various catalytic reactions.