A New Photocatalyst can Efficiently Degrade Broad-Spectrum Antibiotics
Scientists have developed an efficient photocatalyst that can degrade in sulfamethoxazole, a broad-spectrum antibiotic to less hazardous chemicals and reduce health and environmental concerns associated with antibiotic contamination.
Antibiotic contamination has several adverse effects, including antibiotic resistance, ecological impact, human health concerns, etc. Hence, there is a need to find ways to mitigate this environmental issue.
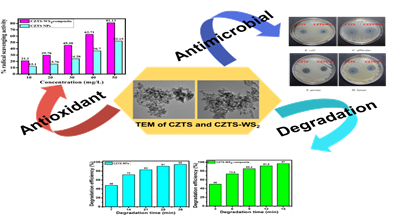
A team of scientists from Institute of Advanced Study in Science and Technology (IASST), Guwahati, an autonomous institute of Department of Science and Technology have synthesized copper zinc tin sulfide Cu2ZnSnS4 (CZTS) nanoparticles (NPs) and copper zinc tin sulfide -tungsten disulfide CZTS-WS2 composite. The team led by Prof. Devasish Chowdhury utilised hydrothermal reaction of zinc chloride, copper chloride, tin chloride and tungsten disulfide forming a composite that is efficient photocatalyst in degrading sulfamethoxazole, an antibiotic.
Broad-spectrum antibiotics like sulfamethoxazole (SMX) have long been used to treat human illnesses like urinary and respiratory tract infections. However, more than 54 % of SMX was released into the environment along with the faeces and urine of the patients.
“CZTS and its nanocomposites are a multifunctional quaternary semiconductor nanomaterial made up of earth-abundant, inexpensive, and non-toxic components possessing remarkable photostability making it extremely valuable in light-harvesting and photocatalyst applications,” said Prof. Chowdhury.
The team consisting of Nur Jalal Mondal, Rahul Sonkar, Mridusmita Barman and Dr. Mritunjoy Prasad Ghosh, established that the CZTS-WS2 composite exhibits good photocatalytic activity for the breakdown of sulfamethoxazole.
The developed catalyst could be recovered and used repeatedly without losing its effectiveness, which is very important from an economic point of view.
Liquid chromatography–mass spectrometry (LC-MS) a popular analytical chemistry technique that can separate and identify the degraded product was used to analyze the intermediates and degraded products of the antibiotics’ degradation reaction. The study published in Journal of Photochemistry & Photobiology A, determined that the majority of intermediates were less hazardous than sulfamethoxazole. In addition, the CZTS-WS2 composite demonstrated more than 80% radical scavenging efficiency and antibacterial capabilities.