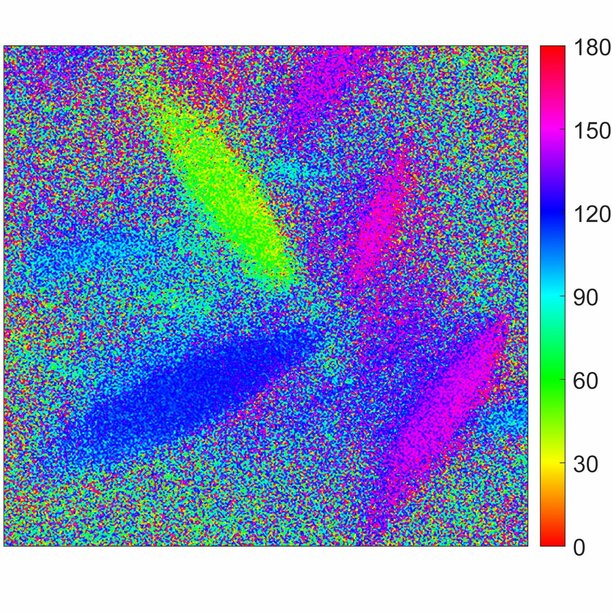
Breakthrough: Synthetic Material Illuminates Liquid Separation Mechanisms
It is quite rare to see a Nature article, with all authors on the paper coming from the same institute. That the recent Nature article by Hailin Fu and Bert Meijer only features TU/e researchers is a testament to the novelty of their findings and the strong collaborative ties at our university. “This is the first time, as far as we know, that someone documents this behavior in synthetic supramolecular polymers,” Fu says. “This synthetic system could become a platform with which we can research organic materials to better understand and manipulate their behavior. This could be the origin story for a potential new avenue of research.”
To understand why Hailin Fu and Bert Meijer are very excited about these findings and their Nature paper, we need to take a few steps back and start at the beginning.
Liquid-liquid phase separation may sound very ‘sciency’ and complicated. But it’s possible to see this separation process at home if you were to place a drop of oil into a glass of water. The liquids won’t mix unless you stir them vigorously. And when left alone, the oil will form separate droplets in the water. In science, this is known as liquid-liquid phase separation.

Cellular phase separation
This process also happens with materials that, contrary to oil, can be dissolved in water. A familiar example to some relates to the separate parts inside an organic tissue cell.
In biology class, we learn that inside the cell there are different areas or parts, known as cell organelles, and each area has different functions and contents. They are all dissolved in the cell plasma, which is water-based. Yet they still cluster together and form separate areas inside the cell plasma solvent. This is also a liquid-liquid phase separation, and that’s the kind that Fu studied, quite serendipitously.
“When I decided to move to the Netherlands to do my research at ICMS, I had a completely different plan,” Fu says. “In the US, where I got my PhD and my first postdoc assignment, I studied polymers mainly.”
“Supramolecular polymers, where polymers form fibers and chains by sticking together without chemical bonds were new to me, and something I wanted to understand better. And, my professor Yao Lin at the University of Connecticut suggested that I go to the Netherlands to follow my academic career.”
“I knew Bert Meijer from a talk he gave in the US and was intrigued. That’s the reason why I’m here today working with Bert.”

Complex to simple
“I started to investigate the well-known liquid-liquid separated aqueous solutions with supramolecular polymers to stabilize it, similar to the ones in Nature and I saw intriguing results. That’s what fascinated me. Bert then suggested switching to a less complex system to study it in more detail.”
“So, it was then that I decided to switch to the UPy solution. In the synthetic materials system I chose to study, I looked at what happens with a droplet of my solution (see UPy highlight) in water,” Fu continues. “And then I saw this strange phenomenon, where there were areas of higher concentration of polymers and areas with a more transparent liquid.”
This is where Meijer adds: “The UPy solution is a suprapolymer system we use for a lot of research at our university, for both solutions, hydrogels, and materials and it’s a system we know very well. You can see this as a blank canvas for us to work on with our research and that of SupraPolix. Patricia Dankers, for instance, uses the UPy platform in her science and as the basis for her work at UPyTher.”
Bye bye droplets! Hello tactoids!
And again in the UPy solution, Fu saw the strange shapes appearing that she saw in her earlier work. Neither Fu nor co-workers at TU/e recognized the shapes, but she was intrigued by their appearance and decided that it was best to figure out what was happening for them to appear in the first place.
The shapes she found, look like elongated grains of rice, called tactoids. Fu: “This is odd because most of the time you will find that when solutions don’t want to mix, they form circular droplets, but not in this case.”
“The advanced imaging setup, with a laser, polarized light and imaging techniques, and a microscope (see highlight), helped me to probe the minute details of the process, and to chart what was happening in the material and in the shapes (tactoids) we saw,” Fu says.
“And the discussions in our group helped too,” notes Fu. “In particular, sitting down and discussing what could be happening, and what this could all mean has helped me a lot. Not only with those who believed I was on to something new but also with the ‘critical voices’ who encouraged me to go back to my results and theories, again and again, to make sure that it all added up.”
“I just never gave up trying to comprehend what was happening in my solutions.”
Meijer adds: “She had me worried for a bit because her research took a long time. We’re always trying to make sure our researcher’s careers stay on track, especially our young colleagues. And I wanted to make sure that she would have something to show for all the work that she’s done at ICMS. I am so happy she succeeded and proud of the work that she did and her perseverance!”
The discovery
As it turns out, Fu discovered a way to make stable, synthetic islands of polymers in a watery solution surrounded by water with a much lower concentration of polymers. And, as far as we now know, this is the first time this has been identified and described as such in synthetic supramolecular polymers.

Meijer: “As Hailin progressed with her research, we started to realize in the team that we had all seen this effect before. Our solutions might have gotten a bit murky when left overnight in the lab.”
“Well, we used to fix that with a vigorous shake in the morning to mix them again and went about our business. None of us thought to study this thoroughly before she did.”
Fu: “That’s also the feedback that I got on the paper when we submitted it. It reads as the meticulous journal of a journey to discovery, and that’s basically what it is. And that is thrilling because it’s a bit like charting a blank space on a map.”
What does it all mean?
That also means that it’s a bit hard to predict whether her results will lead to future applications. It seems likely that her methodology and materials should make a great platform to study the interactions and behavior of organic materials with such synthetic tactoids. For instance, to test methods of drug delivery or cell manipulation.
“And there are also things to research about the supramolecular polymers themselves,” adds Meijer. “We see some fibers align themselves at the borders of the tactoids. They all just align themselves neatly perpendicular to the surface of the glass, and we do not understand that behavior yet. I am so curious to see what causes that.”
“The work also shows that the dogma that natural and synthetic polymers are different is not true anymore. They follow the same physical laws and are all made from the elements C, H, N, O, S, and P. The new systems can be used in connection with biological tissue and can explain many biological systems as they are so much more simple.”

Far from the end of the line
Food for thought, and interesting new avenues of research beckon. Fu will be pursuing them at her new university where she will take the lead as assistant professor focusing on the physical chemistry of supramolecular polymers and macromolecules at Westlake University in China.
“I’m looking forward to those new challenges in the autumn. I hope I can bring the generous and collaborative way of working I experienced in the Netherlands with me. Because my work – our work – is so much better because of it.”
Also at ICMS, plans are being made to continue the research. “There are a couple of ideas, but we’ll see what we can do,” Meijer adds. “This is far from the end of the line for our research. With the techniques and labs we have, it would be a shame to stop now. I, for one, would especially love to know more about the fibers on the surface of the tactoids, their alignment, and the why behind their emergence.”
The future will tell if this serendipitous discovery turns out to have a great impact on the fields of biochemistry and supramolecular materials.