Breakthrough: Synthetic Polymers Offer Promising Solution in Combatting Invasive Fungal Infections
Engineers from UNSW have developed a series of synthetic polymers that offers a promising alternative to current antifungal drugs.
It is estimated that there are around 6.5 million instances of severe fungal infection globally every year, resulting in up to 3.8 million annual deaths. Current commonly used drugs have potential issues with causing toxicity, especially in the liver, while strains of fungi are becoming more resistant to medications due to their prolonged and widespread use, making treatment less effective.
People with underlying health issues or weakened immune systems are at the highest risk of problems with fungal infections, which further complicates the application of current drug formulations.
There has been a recent surge in vulnerable populations, those hospitalised with COVID-19, cancer, or organ transplant patients undergoing treatments. Since some drugs have toxic side effects, it can be difficult to apply them to patients who are already undergoing other strenuous treatments.
Dependent on the species, mortality rates for severe fungal infections can reach 40 per cent and above, even with antifungal intervention, so there is ever-growing urgency in finding more effective solutions.
The development of new antifungal therapeutics has been hindered by the biological similarity between fungi and humans. Although there are a handful novel compounds in clinical trials for which clinical success remains to be seen, no new classes of antifungal drugs have been approved for the treatment of invasive infections over the last two decades, emphasising the need for innovative formulations.
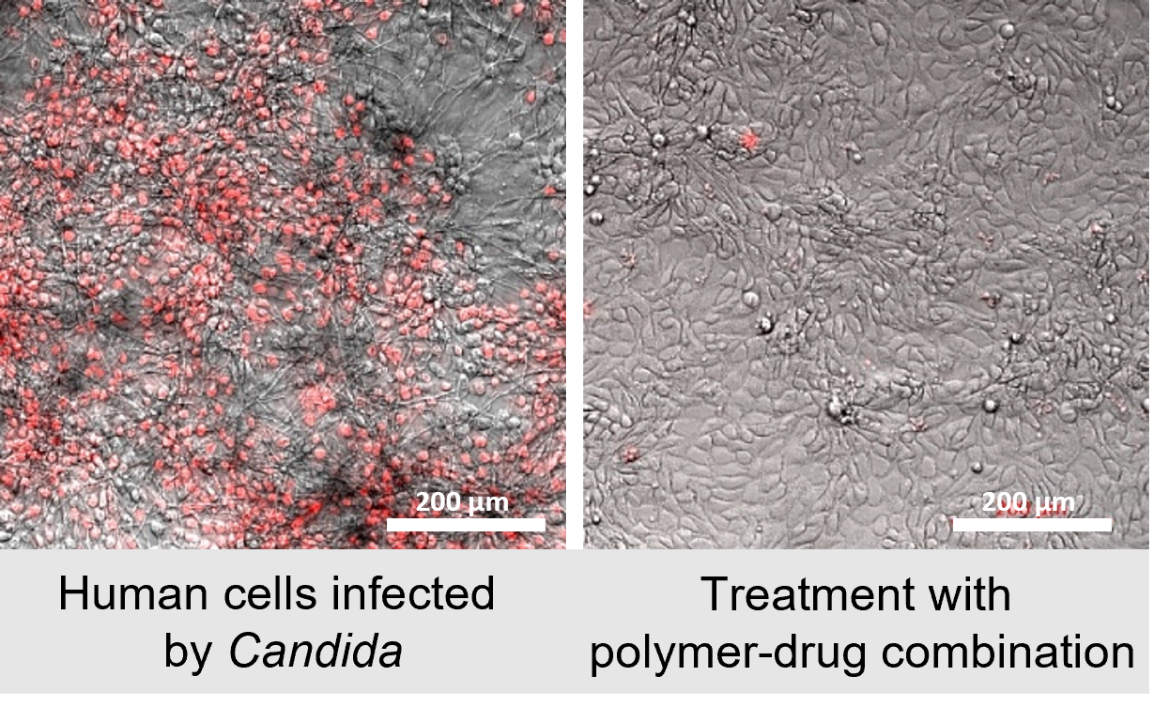
Now, researchers from UNSW have created synthetic polymers that mimic the potent antifungal properties of charged host-defence peptides.
Their findings, recently published in the American Chemical Society (ACS), highlight the development of a range of polymers and show that some were able to outperform current antifungal drugs in terms of therapeutic index, as well as quickly attacking and killing common fungi such as Candida albicans.
The research was led by Professor Cyrille Boyer’s team in the School of Chemical Engineering UNSW, including Dr Nathaniel Corrigan, Dr Daniele Melodia and PhD student Sebastian Schaefer, in collaboration with Dr Megan Lenardon from the UNSW School of Biotechnology and Biomolecular Sciences and Dr Chistopher Tracey from the Nuclear Magnetic Resonance Facility, Mark Wainwright Analytical Centre.
“Natural products are one of the largest sources of antibiotics, like antifungals, and are used to fight harmful invaders like bacteria and fungi,” Prof. Boyer says.
“Antimicrobial peptides, produced naturally, are one defence mechanism by human and other organisms in response to these invaders. While effective, it is challenging to produce antimicrobial peptides on a large scale.
“Different research groups are turning to synthetic polymers, inspired by the properties of antimicrobial peptides, as synthetic polymers are easier to make with diverse compositions, and are more stable.”
These synthetic polymers were prepared by a living radical polymerisation, named photoinduced electron/energy transfer – reversible addition-fragmentation chain-transfer polymerisation (PET-RAFT) developed by Boyer’s group.
PET-RAFT is a variant of RAFT polymerisation invented by CSIRO. This technique enables the preparation of well-defined polymers using low energy light source.
“Existing antifungal drugs have specific fungal targets. Hence, fungi can become resistant to these relatively ‘easily’ compared to antimicrobial peptide-mimicking synthetic polymers,” Prof. Boyer adds.
“As the polymers are a ‘controlled’ mix of compositions, they might target various features of fungal cells at once, which makes it more challenging for the fungal cells to adapt. The same explanation counts for the combination of polymers and existing antifungal drugs, which then again combines multiple modes of action against the fungal cells.”
Impact on public health
“One of the key objectives of this research is to contribute to the development of novel antifungal drug formulations, which are urgently needed to combat the rising incidence of fungal infections worldwide,” says Schaefer.
“By exploring the therapeutic potential of synthetic polymers, researchers aim to unlock new avenues for effectively targeting fungal pathogens and improving patient outcomes.
“Furthermore, the investigation into drug combinations aims to prevent the emergence of drug resistance, a significant concern in the field of antifungal therapy. By optimising drug combinations, researchers seek to enhance the efficacy of treatment while simultaneously reducing the potential for toxic side effects in human patients.”
The research team say the implications of their works extends beyond the realm of antifungal therapy, with potential benefits for a wide range of biomedical applications.
Synthetic polymers offer a versatile platform for drug delivery and therapeutic interventions, with the ability to be easily modified and tailored to specific needs. The results of this study can serve as a guide for other researchers exploring synthetic polymers for biomedical applications, particularly in the antimicrobial field.
“In addition to advancing scientific knowledge, the aim is to develop treatment options that are cost-effective and easy to store, making them accessible in all clinical settings,” adds Schaefer.
“By leveraging the inherent properties of synthetic polymers, researchers hope to create a versatile and scalable solution for addressing the growing burden of fungal infections on public health.”
Existing antifungal drugs have specific fungal targets. Hence, fungi can become resistant to these relatively ‘easily’ compared to antimicrobial peptide-mimicking synthetic polymers.
Enhanced effectiveness
One pivotal aspect of the research involves structural modification of polymers. By interfering with the sequence and architecture of polymers, the team aims to augment their efficacy in combating fungal infections in animal models. This includes exploring the transition from linear chains to circular molecules, potentially enhancing effectiveness.
In addition to structural modification, understanding the intricate interactions between drugs and polymers is important. Through attaching antifungal drugs to polymers and releasing them in the fungal environment, the team strive to harness synergistic effects, thereby amplifying the potency of dual-drug therapy.
Unraveling the mode of action of polymers against fungal cells constitutes another focal point of their investigation. The team endeavors to decipher how polymers exert their effects on fungal cells, offering invaluable insights for shaping future therapeutic approaches.
Equally critical is probing into tolerance and resistance mechanisms. The team aims to discern whether fungal cells can develop tolerance or resistance to synthetic polymers and elucidate the underlying mechanisms. This knowledge is key for devising strategies to circumvent resistance, safeguarding the long-term efficacy of treatment options.
Furthermore, the researchers are committed to testing the spectrum of action of synthetic polymers against a diverse array of pathogenic fungi. While their primary focus lies on Candida species, they aim to broaden the applicability of their findings across various fungal infections. This comprehensive approach underscores their dedication to advancing the field and bolstering the arsenal against fungal pathogens.
The research was also conducted in collaboration with the Australian Centre for NanoMedicine and the Hans Knoell Institute/Leibniz Institute for Natural Product Research and Infection Biology.

