Breakthroughs in Self-Folding Molecules for Nanosized Contrast Agents and Drug Carriers
Self-folding polymers containing gadolinium forming nanosized complexes could be the key to enhanced magnetic resonance imaging and next-generation drug delivery, as demonstrated by scientists at Tokyo Tech. Thanks to their small size, low toxicity, and good tumor accumulation and penetration, these complexes represent a leap forward in contrast agents for cancer diagnosis, as well as neutron capture radiotherapy.
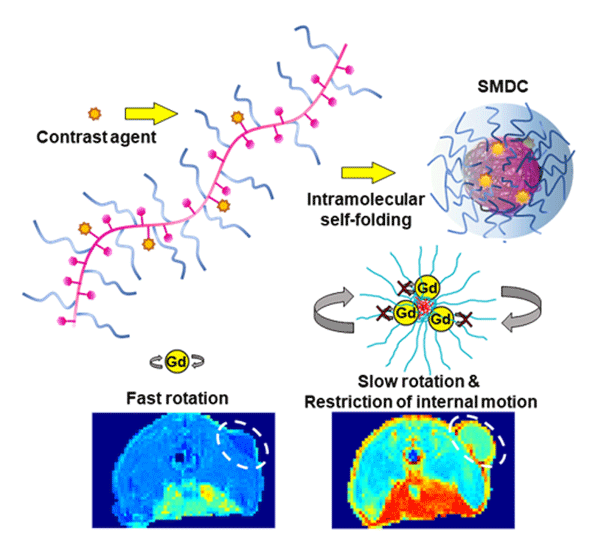
Figure 1. Self-foldToing mechanism leads to enhanced contrast in MRI scans
By incorporating Gd-based contrast agents into a self-folding polymer, researchers from Tokyo Institute of Technology produced nanosized complexes where the motion of Gd ions is restricted. The small size of these complexes led to better accumulation in tumors, and the limited rotation and motion of Gd ions increased the MRI signal, leading to improved contrast.
Magnetic resonance imaging (MRI) is a crucial diagnostic tool for cancer, enabling the capturing of detailed images of soft tissues. To visualize tumors more clearly in MRI scans, doctors usually inject patients with contrast agents. These compounds affect the way nearby hydrogen ions respond to the radiofrequency pulses used in MRI. Ideally, contrast agents should selectively accumulate in tumors and increase their contrast in the MRI scan.
However, despite many research efforts, conventional gadolinium (Gd)-chelate contrast agents are reaching their performance limits. Simply put, achieving an optimal dose in the distribution of Gd-chelates within tumors, healthy tissue, and blood has proven challenging without resorting to excessive Gd doses.
Against this backdrop, a collaborative study by a research team from Tokyo Institute of Technology (Tokyo Tech), National Institutes for Quantum Science and Technology (QST) and Innovation Center of Nanomedicine (iCONM), led by Associate Professor Yutaka Miura of Tokyo Tech, successfully developed a novel NCA with exceptional performance thanks to an innovative molecular design. Their findings were published in the Advanced Science on November 29.
The proposed nano-contrast agent (NCA) is based around the use of Gd as a contrast agent in what the researchers called a “self-folding macromolecular drug carrier (SMDC)”. They incorporated clinically approved Gd-containing chelates into a polymer chain composed of poly(ethylene glycol) methyl ether acrylate (PEGA) and benzyl acrylate (BZA). Since the polymer contained both hydrophilic and hydrophobic segments, it quickly folded itself into a small capsule-like shape when immersed in water, with the hydrophobic segments at the core and the hydrophilic segments at the outer shell.
Using this approach, the researchers could produce SMDC-Gds molecules smaller than 10 nanometers in diameter. Through experiments in mice with colon cancer, they verified that these NCAs not only accumulated better in tumors, but that they were also promptly eliminated from the bloodstream, leading to enhanced MRI performance without toxic effects. “the high accumulation in tumor while quick blood clearance profile of SMDC-Gds allows for the increase in the tumor-to-major organ accumulation ratios as well as minimizing the unnecessary deposition of Gds,” explains Prof. Miura.
Moreover, the team also demonstrated a novel effect that puts SMDC-Gds ahead of existing Gd-chelates. Ideally, the motion of Gd ions should be minimal so that their influence on nearby hydrogen ions is steady and prolonged. In the proposed molecular design, the core/shell structure creates a ‘crowded’ molecular environment that suppresses not only the rotation, but also the segmental and internal motions of Gd ions. The resulting effect is a stronger contrast in MRI images, which will allow for use of alternative elements with safer profiles not only in patients but also environment in future.
It’s worth highlighting that the applications of SMDC-Gds extend beyond MRI. These compounds can be used in neutron capture therapy (NCT), a promising targeted radiotherapy technique in which Gds capture neutrons and release high energy radiations, killing nearby cancer cells. Experiments in mice revealed that NCT following repeated SMDC-Gd injection led to greatly suppressed tumor growth. The team believes the reason for this was the selective accumulation and deep penetration of SMDC-Gds into tumor tissues.
Collectively, the researchers’ collaborative efforts to achieve these findings underscore the potential of SMDCs not only for better MRI diagnostics, but also as effective tools for treating cancer and other diseases. “This study presents further possibilities for exploiting drug delivery using various therapeutic cargos, and we are currently investigating the development of such SMDC systems,” concludes a hopeful Prof. Miura.

