David Julius, Ardem Patapoutian get Nobel Prize 2021 in Medicine
The Nobel Prize in Physiology or Medicine 2021 was awarded jointly to David Julius and Ardem Patapoutian “for their discoveries of receptors for temperature and touch.”
The Nobel Assembly at Karolinska Institutet
has today decided to award
the 2021 Nobel Prize in Physiology or Medicine
jointly to
David Julius and Ardem Patapoutian
for their discoveries of receptors for temperature and touch
Our ability to sense heat, cold and touch is essential for survival and underpins our interaction with the world around us. In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates.
David Julius utilized capsaicin, a pungent compound from chili peppers that induces a burning sensation, to identify a sensor in the nerve endings of the skin that responds to heat. Ardem Patapoutian used pressure-sensitive cells to discover a novel class of sensors that respond to mechanical stimuli in the skin and internal organs. These breakthrough discoveries launched intense research activities leading to a rapid increase in our understanding of how our nervous system senses heat, cold, and mechanical stimuli. The laureates identified critical missing links in our understanding of the complex interplay between our senses and the environment.
How do we perceive the world?
One of the great mysteries facing humanity is the question of how we sense our environment. The mechanisms underlying our senses have triggered our curiosity for thousands of years, for example, how light is detected by the eyes, how sound waves affect our inner ears, and how different chemical compounds interact with receptors in our nose and mouth generating smell and taste. We also have other ways to perceive the world around us. Imagine walking barefoot across a lawn on a hot summer’s day. You can feel the heat of the sun, the caress of the wind, and the individual blades of grass underneath your feet. These impressions of temperature, touch and movement are essential for our adaptation to the constantly changing surrounding.
In the 17th century, the philosopher René Descartes envisioned threads connecting different parts of the skin with the brain. In this way, a foot touching an open flame would send a mechanical signal to the brain (Figure 1). Discoveries later revealed the existence of specialized sensory neurons that register changes in our environment. Joseph Erlanger and Herbert Gasser received the Nobel Prize in Physiology or Medicine in 1944 for their discovery of different types of sensory nerve fibers that react to distinct stimuli, for example, in the responses to painful and non-painful touch. Since then, it has been demonstrated that nerve cells are highly specialized for detecting and transducing differing types of stimuli, allowing a nuanced perception of our surroundings; for example, our capacity to feel differences in the texture of surfaces through our fingertips, or our ability to discern both pleasing warmth, and painful heat.
Prior to the discoveries of David Julius and Ardem Patapoutian, our understanding of how the nervous system senses and interprets our environment still contained a fundamental unsolved question: how are temperature and mechanical stimuli converted into electrical impulses in the nervous system?

Figure 1 Illustration depicting how the philosopher René Descartes imagined how heat sends mechanical signals to the brain.
The science heats up!
In the latter part of the 1990’s, David Julius at the University of California, San Francisco, USA, saw the possibility for major advances by analyzing how the chemical compound capsaicin causes the burning sensation we feel when we come into contact with chili peppers. Capsaicin was already known to activate nerve cells causing pain sensations, but how this chemical actually exerted this function was an unsolved riddle. Julius and his co-workers created a library of millions of DNA fragments corresponding to genes that are expressed in the sensory neurons which can react to pain, heat, and touch. Julius and colleagues hypothesized that the library would include a DNA fragment encoding the protein capable of reacting to capsaicin. They expressed individual genes from this collection in cultured cells that normally do not react to capsaicin. After a laborious search, a single gene was identified that was able to make cells capsaicin sensitive (Figure 2). The gene for capsaicin sensing had been found! Further experiments revealed that the identified gene encoded a novel ion channel protein and this newly discovered capsaicin receptor was later named TRPV1. When Julius investigated the protein’s ability to respond to heat, he realized that he had discovered a heat-sensing receptor that is activated at temperatures perceived as painful (Figure 2).
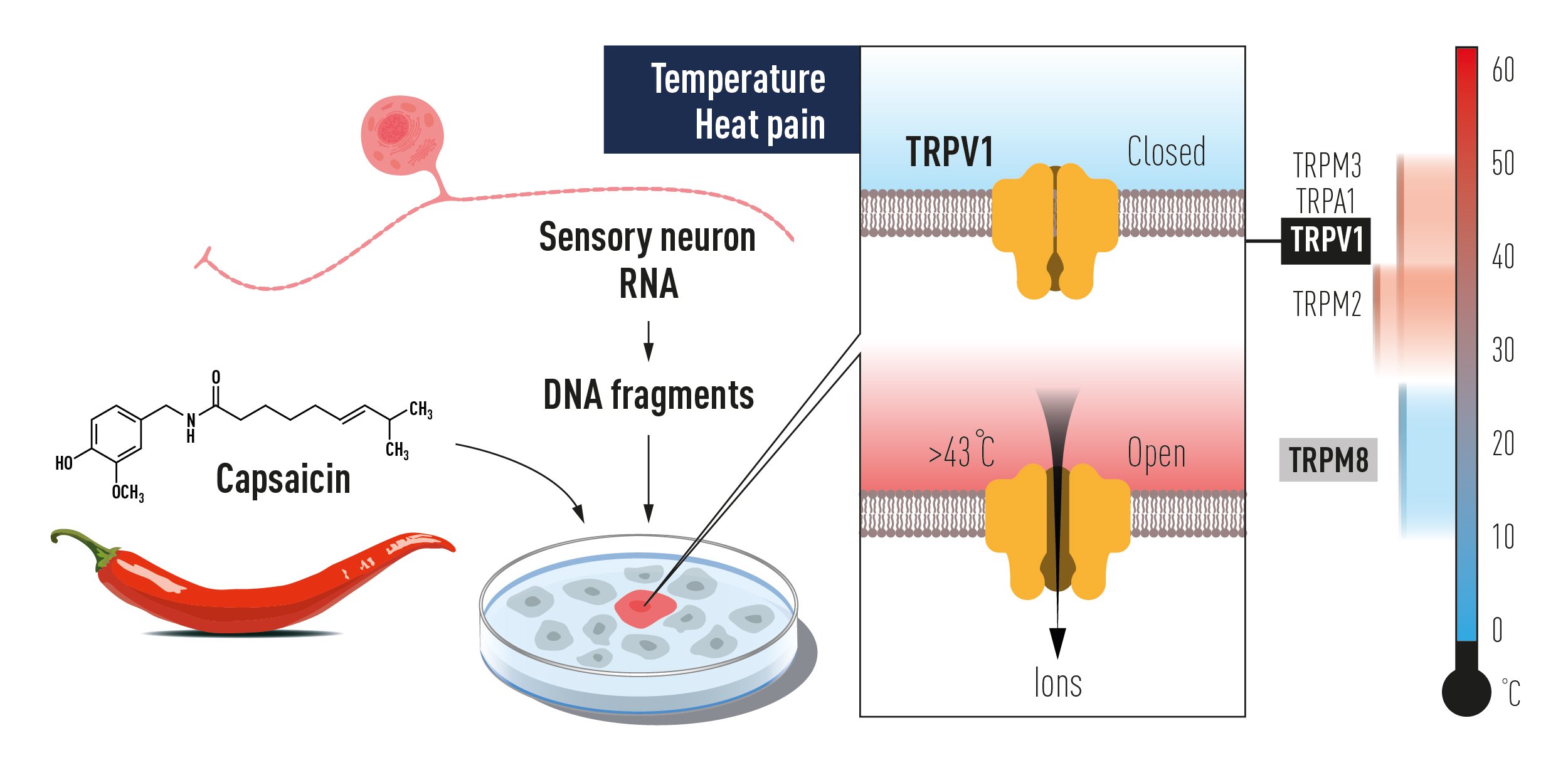
Figure 2 David Julius used capsaicin from chili peppers to identify TRPV1, an ion channel activated by painful heat. Additional related ion channels were identified and we now understand how different temperatures can induce electrical signals in the nervous system.
The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors. Independently of one another, both David Julius and Ardem Patapoutian used the chemical substance menthol to identify TRPM8, a receptor that was shown to be activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures. Many laboratories pursued research programs to investigate the roles of these channels in thermal sensation by using genetically manipulated mice that lacked these newly discovered genes. David Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system.
Research under pressure!
While the mechanisms for temperature sensation were unfolding, it remained unclear how mechanical stimuli could be converted into our senses of touch and pressure. Researchers had previously found mechanical sensors in bacteria, but the mechanisms underlying touch in vertebrates remained unknown. Ardem Patapoutian, working at Scripps Research in La Jolla, California, USA, wished to identify the elusive receptors that are activated by mechanical stimuli.
Patapoutian and his collaborators first identified a cell line that gave off a measurable electric signal when individual cells were poked with a micropipette. It was assumed that the receptor activated by mechanical force is an ion channel and in a next step 72 candidate genes encoding possible receptors were identified. These genes were inactivated one by one to discover the gene responsible for mechanosensitivity in the studied cells. After an arduous search, Patapoutian and his co-workers succeeded in identifying a single gene whose silencing rendered the cells insensitive to poking with the micropipette. A new and entirely unknown mechanosensitive ion channel had been discovered and was given the name Piezo1, after the Greek word for pressure (í; píesi). Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes (Figure 3).
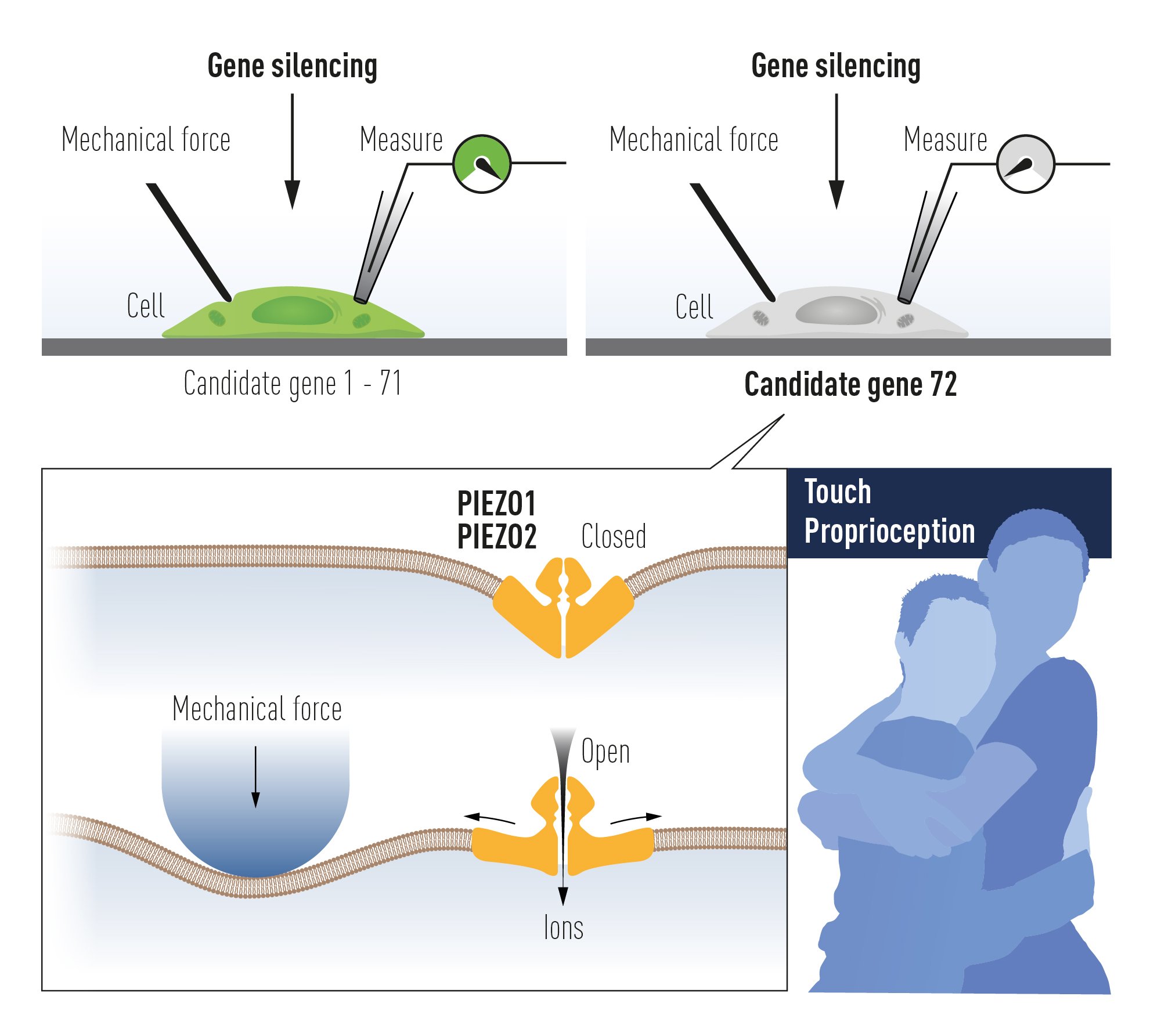
Figure 3 Patapoutian used cultured mechanosensitive cells to identify an ion
channel activated by mechanical force. After painstaking work, Piezo1 was
identified. Based on its similarity to Piezo1, a second ion channel was found
(Piezo2).
The breakthrough by Patapoutian led to a series of papers from his and other groups, demonstrating that the Piezo2 ion channel is essential for the sense of touch. Moreover, Piezo2 was shown to play a key role in the critically important sensing of body position and motion, known as proprioception. In further work, Piezo1 and Piezo2 channels have been shown to regulate additional important physiological processes including blood pressure, respiration and urinary bladder control.
It all makes sense!
The groundbreaking discoveries of the TRPV1, TRPM8 and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us. The TRP channels are central for our ability to perceive temperature. The Piezo2 channel endows us with the sense of touch and the ability to feel the position and movement of our body parts. TRP and Piezo channels also contribute to numerous additional physiological functions that depend on sensing temperature or mechanical stimuli. Intensive ongoing research originating from this year’s Nobel Prize awarded discoveries focusses on elucidating their functions in a variety of physiological processes. This knowledge is being used to develop treatments for a wide range of disease conditions, including chronic pain (Figure 4).
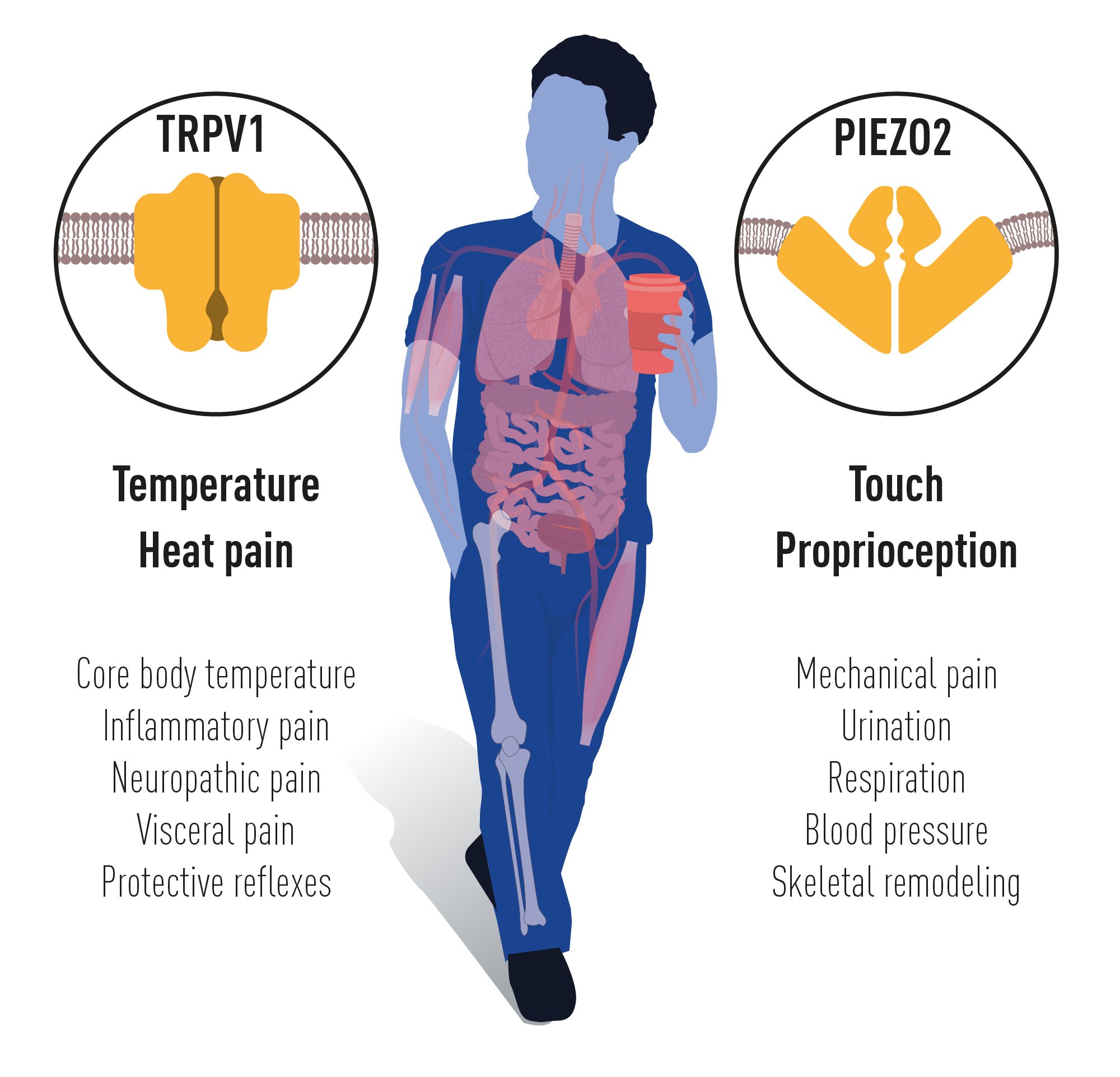
Figure 4 The seminal discoveries by this year’s Nobel Prize laureates have explained how heat, cold and touch can initiate signals in our nervous system. The identified ion channels are important for many physiological processes and disease conditions.
Key publications
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997:389:816-824.
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998:21:531-543.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000:288:306-313
McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002:416:52-58
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 2002:108:705-715
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010:330: 55-60
Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014:516:121-125
Woo S-H, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A. Piezo2 is the principal mechonotransduction channel for proprioception. Nature Neuroscience 2015:18:1756-1762
David Julius was born in 1955 in New York, USA. He received a Ph.D. in 1984 from University of California, Berkeley and was a postdoctoral fellow at Columbia University, in New York. David Julius was recruited to the University of California, San Francisco in 1989 where he is now Professor.
Ardem Patapoutian was born in 1967 in Beirut, Lebanon. In his youth, he moved from a war-torn Beirut to Los Angeles, USA and received a Ph.D. in 1996 from California Institute of Technology, Pasadena, USA. He was a postdoctoral fellow at the University of California, San Francisco. Since 2000, he is a scientist at Scripps Research, La Jolla, California where he is now Professor. He is a Howard Hughes Medical Institute Investigator since 2014.
Illustrations: © The Nobel Committee for Physiology or Medicine. Illustrator: Mattias Karlén

