Enhancing Thermal Energy Storage by Improving the Properties of Sweeteners
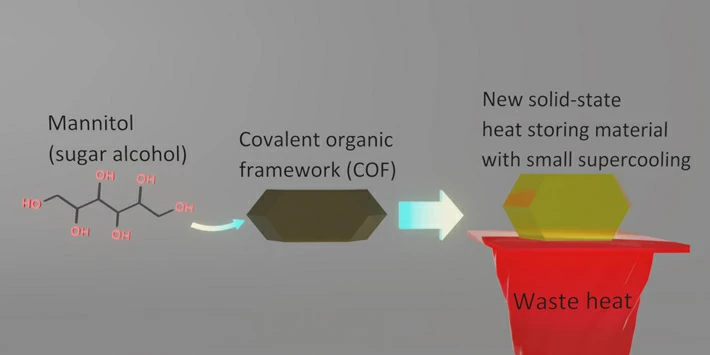
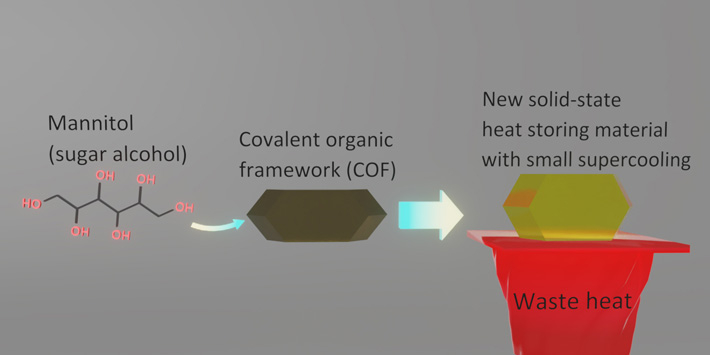
A solution to enhance the thermal energy storage of sugar alcohols has been developed by researchers from Tokyo Tech. They achieved this by confining sugar alcohols, commonly used sweeteners, in the pores of a covalent organic framework (COF), effectively resolving the long-standing problem of supercooling that degrades the stored thermal energy. This innovative material can store-and-release heat much more efficiently, potentially opening doors to novel, eco-friendly solid-state heat storage materials.
As we seek more efficient utilization of waste thermal energy, use of “phase change materials (PCMs)” is a good option. PCMs have a large latent heat capacity and the ability to store-and-release heat as they change from one state of matter to another. Among many PCMs, sugar alcohols (SAs), a class of organic compounds commonly used as sweeteners, stand out due to their low cost, non-toxic, non-corrosive, and biodegradable nature. In particular, SAs generally have their melting point in 100—200 ℃, which is an important temperature range where a huge amount of waste heat exists but is currently being discarded in our world.
However, SAs usually suffer from the issue of supercooling where, instead of solidifying, they remain in a liquid state even at temperatures well below the melting point. The supercooling degrades the quality (or “exergy“) of stored thermal energy because thermal energy at lower temperature has less usefulness. (Note: Thermal energy at room temperature is totally useless, no matter how much of it exists.)
Now, in a new study, researchers from Tokyo Institute of Technology (Tokyo Tech) led by Professor Yoichi Murakami have discovered that confining SAs in covalent organic framework (COF) crystals effectively resolves the issue of supercooling. Their findings, published in the journal Materials Horizons, have the potential to revolutionize SAs as heat-storage materials.
Dr. Murakami, who is a Professor at the Laboratory for Zero-Carbon Energy at Tokyo Tech, explains, “We propose a new materials concept with which the stored thermal energy can be retrieved at a much higher temperature than before, by largely mitigating the long-standing issue of supercooling that degrades the stored thermal energy. We have created a new class of solid-state PCMs based on abundant, non-toxic, and low-cost SAs.”
Normally, pure D-mannitol (Man), one of SAs, has a melting point of 167 ℃, but it usually solidifies at random temperatures around 80—120 ℃, which is a large supercooling of about 47—87 ℃. To resolve this issue, the researchers introduced Man into the crystals of COF-300, one of the most typical COFs. They discovered that while the melting of Man confined in the COF occurred at around 150—155 ℃, the freezing of the Man confined in the COF reproducibly occurred in the slightly lower temperature range of 130—145 ℃. Therefore, the supercooling has been suppressed to only 10—20 ℃, much smaller than the previous supercooling of about 47—87 ℃.
“These results indicate that the fusion–freezing cycles of the Man–COF composite occur within a narrow temperature range of 130—155 ℃ without large or random supercooling,” says Prof. Murakami, highlighting the discovered effect of the COF confinement.
According to their published paper, earlier works confined SAs in rigid inorganic porous materials such as nanoporous silica and alumina to form solid-state PCMs, but they failed to resolve the supercooling issue of SAs. COFs are not only flexible porous materials but also have much smaller pores (in the order of single-nanometer scale) than those of previous inorganic nanoporous materials. The present study is expected to pave the way for the new class of solid-state heat storage materials based on green and low-cost SAs for efficient thermal energy storage.