New Study Provides Insights into Debate on Two Types of Shoulder Replacement Surgery for Osteoarthritis
A new study has provided valuable insights into the ongoing debate surrounding two types of shoulder replacement surgery: reverse total shoulder replacement and anatomical total shoulder replacement as a treatment for patients with osteoarthritis.
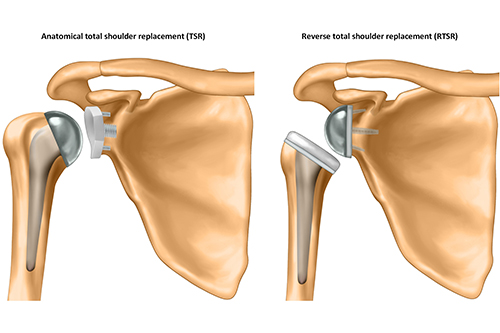
The research, led by the University of Oxford and involving researchers from the University of Bristol, has found that reverse total shoulder replacements (RTSR) provide similar long-term outcomes to traditional anatomical total shoulder replacements (TSR) for patients aged 60 years or older with osteoarthritis (OA) and intact rotator cuff tendons.
The study published in the BMJ today [1 May] was funded by the National Institute for Health and Care Research (NIHR).
Shoulder OA is a common and debilitating condition, and shoulder replacement surgery is an effective treatment option for end-stage disease. TSR has long been considered the gold standard for treating patients with OA and intact rotator cuff tendons. However, the RTSR has surged in popularity since 2008 in the UK. Originally designed for a completely different surgical indication, it is now often used instead of TSR in this patient group.
This shift in practice is growing despite a lack of supporting evidence, and in 2020, the National Institute for Health and Care Excellence (NICE) identified this as a key research priority. Researchers from NDORMS, University of Oxford, and involving experts from the University of Bristol set out to provide high-quality evidence to help address this uncertainty.
Epaminondas Markos Valsamis, NIHR Doctoral Research Fellow at the University of Oxford and lead author, explained: “In recent years, the use of RTSR has increased, even for patients with intact rotator cuffs – a group traditionally treated with TSR. But treatment choices are being made without any good evidence, leading to concerns from healthcare agencies and patients about which procedure is the safest and most effective option.”
Dr Adrian Sayers, Senior Research Fellow in the Bristol Medical School: Translational Health Sciences (THS) and co-author, added: “This work shows the tremendous potential of routinely collected data in answering questions that are important to patients in a timely manner. It is reassuring for patients and surgeons to know that either reverse or traditional anatomic shoulder replacement for osteoarthritis is a safe and effective procedure. This question was asked and answered in a fraction of the time and cost of conventional randomised clinical studies.”
The research team conducted a population-based cohort study using linked data from the National Joint Registry and NHS Hospital Episode Statistics for England. Over 12,000 patients aged 60 years or older who underwent RTSR or TSR for OA with intact rotator cuff tendons between 2012 and 2020 were included in the study.
The researchers compared the outcomes of patients for each of the two types of procedures, focusing on factors such as revision surgery, serious adverse events, reoperations, hospital stay duration, and lifetime costs to the healthcare system.
The findings revealed that while TSR had a higher risk of revision surgery in the first three years after surgery, there was no important difference in the longer term, and both procedures were equally safe for patients.
“By the end of the study period we found no ‘clinically important’ difference in any outcome”, said Epaminondas. “This provides reassurance to patients and surgeons that RTSR is an acceptable alternative for this patient group, and we found no evidence to change the growing surgical trend of offering RSTR to them.”
While further research is needed to explore functional outcomes and to inform a full cost-effectiveness comparing RTSR and TSR, this study provides valuable insights that can help guide clinical practice by supporting patients and surgeons to make more informed decisions about the best treatment options in order to optimise patient outcomes.
The study was supported by the NIHR Oxford Biomedical Research Centre (BRC), NIHR Bristol Biomedical Research Centre (NIHR Bristol BRC) and the National Joint Registry.