POSTECH, KRICT, and LG Energy Solution Unravel Mechanism for Stabilizing Cathode Doping in Electric Vehicle Batteries
Professor Si-Young Choi, and So-Yeon Kim and Yu-Jeong Yang, PhD candidates, from the Department of Materials Science and Engineering at Pohang University of Science and Technology (POSTECH) along with Dr. Sungho Choi from the Korea Research Institute of Chemical Technology (KRICT) and Dr. Sora Lee and Chiho Jo from LG Energy Solution have made a breakthrough in understanding the stabilization mechanism of surface structures in high-capacity, high-nickel Cathode materials through single-element doping*1 in their collaborative research through quantitative analysis. Their work was published in “Chemical Engineering Journal”, an international journal in the field of chemical engineering.
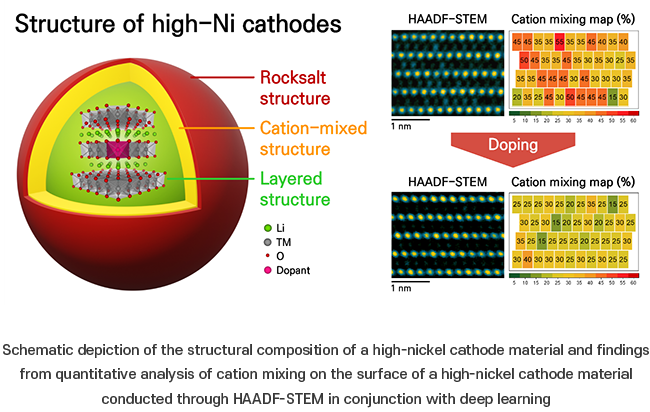
In the quest to extend the driving range of electric vehicles, there’s a growing need for cathode materials with a higher capacity to store more power. Nickel (Ni) is widely used in electric vehicle batteries due to its high energy density. High-nickel compounds like LiNi0.8Co0.1Mn0.1O2 are common cathode materials, boasting substantial nickel content. However, as the concentration of nickel rises, a concerning phenomenon emerges: nickel ions infiltrate the lithium layer by exchanging positions with similarly sized nickel and lithium ions along certain surfaces. This excessive cation mixing has been linked to diminished battery performance.
To address this issue, recent research has focused on incorporating metal ions as dopants*2. These metal cations are placed within the transition metal or lithium layers of high-nickel cathode materials. Precise the doping sites is crucial to understand their effect on the structural stability of the cathode materials. However, the small quantity of metal cations added to enhance cathode performance poses challenges in pinpointing their exact locations and studying the stabilization mechanism.
In this research, the team developed a deep learning AI technique to quantitatively analyze cation mixing using atomic structure images. They combined this approach with atomic-scale electron microscopy (HAADF-STEM*3), allowing them to visualize, for the first time, the location of aluminum (Al), titanium (Ti), and zirconium (Zr) metal dopants at sub-molar concentrations (mol %) in high-nickel cathode materials. Through this method, they were able to examine how these dopants affect the surface structure and electrochemical properties of the cathode material.
The examination revealed that the introduction of three metal cations into the transition metal layer fortified the bonds between nickel and oxygen atoms, thereby curbing cation mixing and enhancing structural stability. Among aluminum, titanium, and zirconium, all contributed to increased discharge capacity and retention in the high-capacity nickel cathode material with titanium exhibiting the most pronounced effect. This marks the first quantitative assessment and analysis of cation mixing defects, a domain previously restricted to qualitative examination.
POSTECH Professor Si-Young Choi who led the research stated, “We developed a deep learning technology for the quantitative analysis of cation mixing in high-nickel cathode materials, enhancing the effectiveness of atomic-scale structural analysis.” He expressed expectation by saying, “Our aim is to lay the groundwork for technologies that analyze highly sensitive materials, thereby advancing the understanding of performance enhancement mechanisms for next-generation cathode materials.”
The research was conducted with support from the Nanomaterial Technology Development Program of the Ministry of Science and ICT, the Dual-Use Technology Projects of the Ministry of Trade, Industry and the Energy and Defense Acquisition Program Administration, the National research Facilities & Equipment Center of the Korea Basic Science Institute under the Ministry of Education, and LG Energy Solution.