Research Group Draws Feedback Loop Between Cancer Cells And Macrophages Drives Gastric Cancer Liver Metastasis
Metastasis is a major cause of death for gastric cancer patients. The liver is the most common site for distant metastasis of gastric cancer. However, the mechanisms underlying gastric cancer liver metastasis remain largely unknown and the clinical treatment options are very limited. Therefore, it is in urgent need to systematically identify liver metastasis-related genes and discover new therapeutic targets for gastric cancer patients with liver metastasis
Recently, the research group led by Prof. ZHOU Tianhua and Prof. ZHUO Wei from the School of Basic Medicine, Zhejiang University School of Medicine published a research paper entitled “MAPK4 silencing in gastric cancer drives liver metastasis by positive feedback between cancer cells and macrophages” in Experimental & Molecular Medicine. For the first time, they uncovered MAPK4 acts as a metastasis suppressor in gastric cancer, and MAPK4 downregulation mediates a new positive feedback loop between gastric cancer cells and macrophages in primary tumor to facilitate liver metastasis of gastric cancer cells.
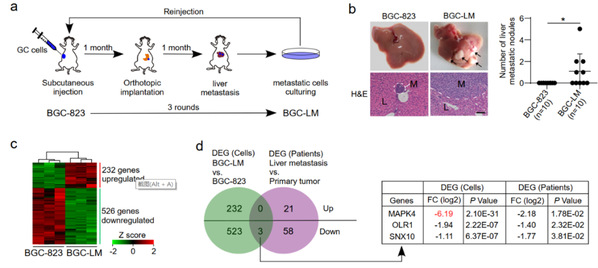
Researchers systematically screened liver metastasis-related genes in vivo using an orthotopic xenograft mouse model. After three rounds of selection, a highly liver metastatic population of cells was obtained. Further transcriptome profiling analysis showed that mitogen activated protein kinase 4 (MAPK4) was the most significantly downregulated gene in high liver metastatic cells. And MAPK4 knockdown could significantly promote liver metastasis of gastric cancer cells in orthotopic mouse models. The clinical correlation analysis showed that gastric cancer tissues had significantly lower expression levels of MAPK4 than adjacent nontumor tissues and the downregulation of MAPK4 was associated with the poor prognosis of gastric cancer patients.
Interestingly, the research group found that MAPK4 depletion inhibited cell invasion in vitro but promoted cell invasion in vivo, implying that the function of MAPK4 in gastric cancer metastasis may depend on tumor microenvironment. Further studies revealed that MAPK4 could affect the cytokine secretion profile of tumor cells. The downregulation of MAPK4 significantly increased the secretion of macrophage migration inhibitory factor (MIF) to activate tumor-associated macrophage (TAM) polarization, which eventually promotes liver metastasis of gastric cancer cells. Further mechanism studies showed that MAPK4 could phosphorylate MIF to increase its ubiquitination and degradation in gastric cancer cells.
In turn, TAMs were found to activate EMT to inhibit MAPK4 expression in gastric cancer cells, which forms a positive feedback loop between gastric cancer cells and macrophages mediated by MAPK4 downregulation to drive liver metastasis of gastric cancer cells in the tumor microenvironment.
Taken together, the study systematically screens the key regulatory genes of gastric cancer liver metastasis in vivo using the orthotopic xenograft mouse model and elucidates MAPK4 may be a key suppressor gene of liver metastasis in gastric cancer. This work reveals a new tumor cell-macrophage interaction mediated by the MAPK4 downregulation in promoting liver metastasis of gastric cancer cells, which provides potential targets for the clinical treatment and prognosis of gastric cancer liver metastasis.