Researchers Develop Embryo-Like Model to Simulate Early Human Blood Production
University of Pittsburgh researchers have developed a new embryo-like model derived from adult cells that replicates key features of early human development, including the generation of blood cells.
Described today in Nature, the new heX-Embryoid model provides a unique window into early human development, which has been shrouded in mystery because of ethical and technical challenges of studying this period of life. HeX-Embryoids, which do not use fetal tissue and cannot develop into an embryo, could enhance research on genetic diseases and infertility and make cells to replace or repair tissues for regenerative medicine applications.
 “Human embryos — unlike those in other species, including some of our closest primate relatives — embed themselves into the uterine wall to proceed with development. Because the embryo is smaller than the tip of a sewing needle and hidden from view, these early stages are difficult to study,” said senior author Mo Ebrahimkhani, M.D., associate professor in the Department of Pathology, the Pittsburgh Liver Institute and the Department of Bioengineering at Pitt. “Our embryo-like model will unlock this ‘black box’ of human development, which could help solve the mystery of why about 60% of pregnancies fail in the first two weeks — before the mother even misses a menstrual period — and pave the way for new therapies.”
“Human embryos — unlike those in other species, including some of our closest primate relatives — embed themselves into the uterine wall to proceed with development. Because the embryo is smaller than the tip of a sewing needle and hidden from view, these early stages are difficult to study,” said senior author Mo Ebrahimkhani, M.D., associate professor in the Department of Pathology, the Pittsburgh Liver Institute and the Department of Bioengineering at Pitt. “Our embryo-like model will unlock this ‘black box’ of human development, which could help solve the mystery of why about 60% of pregnancies fail in the first two weeks — before the mother even misses a menstrual period — and pave the way for new therapies.”
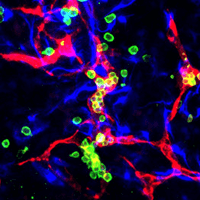
Remarkably, the heX-Embryoid models formed structures similar to the first sites to produce blood cells that support the developing embryo called blood islands. The researchers also detected progenitors of red blood cells, platelets and different types of white blood cells. According to Ebrahimkhani, the generation of blood cells is a key advance of this embryo model that pushes the field forward.
 “We were able to model something extremely similar to the earliest stages of blood production in humans,” said Ebrahimkhani, who is also a member of the Pittsburgh Liver Research Center and the McGowan Institute of Regenerative Medicine of Pitt and UPMC. “This is exciting because there are extensive possibilities to apply this model to better understand how blood is formed and develop better methods for growing cells for blood transfusions, novel cell therapies and hematopoietic stem cell transplants.”
“We were able to model something extremely similar to the earliest stages of blood production in humans,” said Ebrahimkhani, who is also a member of the Pittsburgh Liver Research Center and the McGowan Institute of Regenerative Medicine of Pitt and UPMC. “This is exciting because there are extensive possibilities to apply this model to better understand how blood is formed and develop better methods for growing cells for blood transfusions, novel cell therapies and hematopoietic stem cell transplants.”
To develop heX-Embryoids, the researchers started with induced pluripotent stem cells (iPSCs), which are generated from adult cells that have been reverted to a state where they can develop into any other cell. Then they programmed the iPSCs with a genetic circuit that directs early tissue development, which is only switched on by a chemical called doxycycline. When these engineered iPSCs are mixed in a lab dish with standard iPSCs and induced by adding doxycycline, the engineered cells grow and trigger the standard iPSCs to organize into three-dimensional structures that resemble certain features of an embryo.
In normal embryonic development, cells repeatedly sort and divide to eventually form distinct sections: the trophoblast, which will become the placenta, an extra-embryonic cell layer that produces the nutrient-providing yolk sac and the embryonic layer that will give rise to the embryo itself and the amniotic sac that protects the developing embryo.
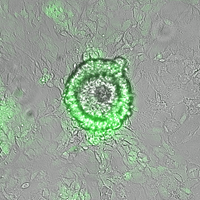
Like an embryo, heX-Embryoids have embryonic tissue and a yolk sac structure. The tissue remains anchored to the lab dish as it grows, forming a large sheet of yolk sac with dozens of embryoids sitting side by side.
 “The yolk sac doesn’t contribute directly to making cells that form the embryo, but it’s a really important tissue because it’s responsible for nourishment and influencing where the head and tail of the embryo will be positioned,” said lead author Joshua Hislop, a graduate student in Ebrahimkhani’s lab at Pitt. “Other embryo-like models have had very limited differentiation of yolk sac tissue, so our model offers a unique opportunity to robustly follow this structure and study events like blood development.”
“The yolk sac doesn’t contribute directly to making cells that form the embryo, but it’s a really important tissue because it’s responsible for nourishment and influencing where the head and tail of the embryo will be positioned,” said lead author Joshua Hislop, a graduate student in Ebrahimkhani’s lab at Pitt. “Other embryo-like models have had very limited differentiation of yolk sac tissue, so our model offers a unique opportunity to robustly follow this structure and study events like blood development.”
HeX-Embryoids do not contain the placenta-forming trophoblast layer, and the yolk sac is open, not a closed cavity. The lack of these features prevents embryoids becoming a true embryo or having the potential to be implanted to develop completely.
Because heX-Embryoids are derived from reprogrammed adult skin cells, they could theoretically be made from any individual, allowing researchers to study diverse genetic backgrounds.
An important advantage of the heX-Embryoid system over other embryo-like models is that it self-organizes as it grows from the two-dimensional lab dish, uses standard growth media and is switched on by a single chemical, rather than relying on a complicated cocktail of growth factors that can be difficult to replicate. According to Ebrahimkhani, this unique approach means that heX-Embryoids can be easily stored, shipped and grown in different labs with a high level of efficiency.
“For a model to be adopted by the scientific community and do its job of contributing to new discoveries, it must be efficient,” said Ebrahimkhani. “For example, it will be very difficult to make progress in researching miscarriage if the model itself fails most of the time. Our heX-Embryoid model overcomes this problem.”
 Other authors on the study were Kamyar Keshavarz F., Rayna Schoenberger, Ryan LeGraw, Jeremy Velazquez, Ph.D., Tahere Mokhtari, Mohammad Naser Taheri, Matthew Rytel, Simon Watkins, Ph.D., Donna Stolz, Ph.D., and Samira Kiani, M.D., all of Pitt; Qi Song, Ph.D., Amir Alavi, Ph.D., and Ziv Bar-Joseph, Ph.D., all of Carnegie Mellon University; Susana Chuva de Sousa Lopes, Ph.D., of Leiden University Medical Center; and Berna Sozen, Ph.D., of Yale University.
Other authors on the study were Kamyar Keshavarz F., Rayna Schoenberger, Ryan LeGraw, Jeremy Velazquez, Ph.D., Tahere Mokhtari, Mohammad Naser Taheri, Matthew Rytel, Simon Watkins, Ph.D., Donna Stolz, Ph.D., and Samira Kiani, M.D., all of Pitt; Qi Song, Ph.D., Amir Alavi, Ph.D., and Ziv Bar-Joseph, Ph.D., all of Carnegie Mellon University; Susana Chuva de Sousa Lopes, Ph.D., of Leiden University Medical Center; and Berna Sozen, Ph.D., of Yale University.
This research was supported by the National Heart, Lung, and Blood Institute (R01 HL141805), the National Science Foundation (#2134999), the National Institutes of Health (1R01GM122096, OT2OD026682, 1U54AG075931, 1U24CA268108, T32 EB001026 and 1S10OD019973-01), the National Institute of Biomedical Imaging and Bioengineering (EB028532), the Pittsburgh Liver Research Center (NIHNIDDK P30DK120531), the Pitt Department of Pathology startup fund, the Pitt Center for Biological Imaging and the Pitt Flow Cytometry Core facilities.