Revolutionizing Cancer Therapy: Innovation Inspired by Freeze-Dried Fruits
Freeze-drying technique, commonly used for preparation of snacks from fruits and vegetables, is now utilized for generating therapeutic materials for cancer treatment. The freeze-drying process could maintain the color, flavor, and nutritional content of food by removing water from solid to gas phase directly under reduced pressure and low temperature. It is being harnessed to preserve active components and structures of human tissues as drug depots.

Freeze-dried fruits and vegetables.
Led by Professor GU Zhen from the College of Pharmaceutical Sciences at Zhejiang University, a research team proposed a novel approach involving the lyophilization of lymph nodes from patients to serve as bioactive materials for loading and delivering cell therapeutics. This innovative strategy demonstrated promising results in animal models, showing persistent inhibition of postoperative recurrence in malignant tumors. This out-of-the-box research was recently published in Nature Materials.
While therapies utilizing chimeric antigen receptor (CAR) T cells have shown remarkable success in treating certain blood cancers, their efficacy against solid tumors has been limited by various challenges. The dense extracellular matrix of solid tumors often hinders the infiltration of CAR T cells, while the hostile tumor microenvironment can also render dysfunction of these cells.

Study team led by GU Zhen.
Lymph nodes play a critical role in the body’s immune response, serving as central hubs for the activation and expansion of immune cells. Within lymph nodes, the specialized environment supports immune cell function and facilitates the recruitment and survival of these cells. In clinical practice, surgeons frequently remove regional lymph nodes during cancer surgery to assess metastases.
We employed freeze-drying techniques to transform lymph nodes into a biocompatible and autologous material, explained Gu. By preserving the structural integrity and bioactive molecules within lymph nodes, we aim to improve the effectiveness of CAR T cell therapies while reducing the risk of rejection associated with foreign carriers.
The drug-loaded lyophilized lymph nodes can be implanted into the surgical cavity following tumor resection, serving as a reservoir for CAR-T cells, Gu continued. This reservoir ensures a continuous release of CAR T cells, effectively targeting residual tumor cells and preventing postoperative recurrence.
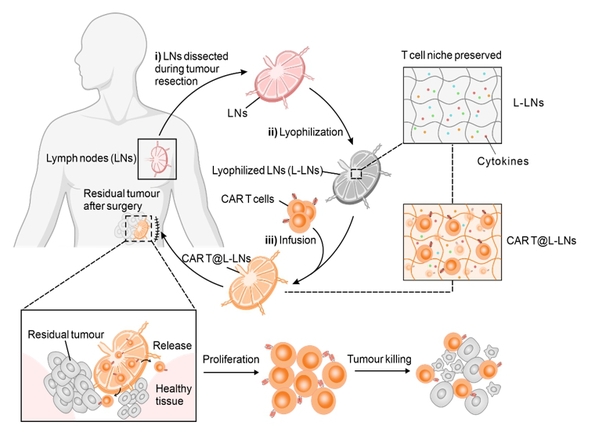
Schematic showing the implantation of patient-derived lyophilized lymph nodes loaded with CAR T cells in the postsurgical tumor bed.
When CAR T cells were loaded inside freeze-dried lymph nodes, they multiplied 3.5 times faster than when they were in artificial hydrogels (the traditional CAR T cells delivery carrier), co-cultured with tumor cells over a 3-day period. Additionally, after 7 days of culture, lyophilized lymph nodes could help CAR T cells memorize the tumor cells, which was important for long-lasting effectiveness.
Utilizing lyophilized lymph nodes resulted in better efficacy than using hydrogels in tumor models, especially derived from patient tumors. The innovative treatment showed superior anti-tumor effects, increased intratumoral activation of CAR T cells, and elevated production of anti-tumor cytokines compared to thehydrogel group receiving the same dosage, explained Hongjun Li, Ph.D., the co-lead author of this study from the College of Pharmaceutical Sciences and Jinhua Institute of Zhejiang University.

Lyophilization of lymph nodes.
ZHAO Peng, M.D., another corresponding author from the First Affiliated Hospital, Zhejiang University School of Medicine, envisioned, In the future, the entire formulation could be prepared within just a few hours, or even during surgery.
Freeze-drying technology can be applied to other tissues and organs to create bioactive materials with unique properties, stated CHEN Dong, M.D., one of the first authors from the First Affiliated Hospital, Zhejiang University School of Medicine.

