Study Analyses Conformational Cycle Of Human Polyamine Transporter
Polyamines are the compounds containing two or more amino groups. They are vital for the viability and growth of cells, and their homeostasis depends largely on two systems: the metabolic system, which regulates the synthesis and degradation of polyamines, and the transport system, which determines the distribution of cellular polyamines. As an essential compartment in the polyamine transport system, lysosomes play a critical role in balancing polyamine concentrations. The lysosomal transporter ATP13A2 can transport polyamines from lysosomes to cytosols. Mutations in the human ATP13A2 gene are linked to hereditary diseases such as Kufor–Rakeb Syndrome (KRS; a type of juvenile-onset Parkinson ‘s disease) and autosomal recessive spastic paraplegia 78. However, the molecular mechanism of ATP13A2 in polyamine homeostasis is still unclear.
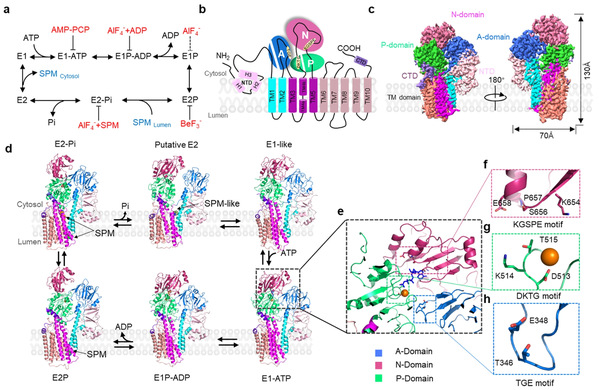
A research team led by Research Fellow WANG Yong from the Zhejiang University College of Life Sciences and Prof. LIU Zhongmin from the Southern University of Science and Technology conducted a study on this topic, and their findings were published in an article entitled Conformational cycle of human polyamine transporter ATP13A2 in the journal Nature Communications on April 8, 2023. They used cryo-electron microscopy (cryo-EM) to capture the high-resolution atomic structures of ATP13A2 in six intermediate states, thereby revealing a nearly complete atomic-level conformational cycle spanning the well-known Post-Albers cycle. By integration of high-resolution structures, biochemical assays, mass spectrometry, and molecular dynamics (MD) simulations, they elucidated how human ATP13A2 recruits, translocates, and releases polyamine substrates, thus shedding light on the pathogenic mechanism of hATP13A2 in neuronal diseases.
Polyamine recruitment. The first step in polyamine transport by hATP13A2 is the recruitment of polyamines to the transporter from the luminal side. The researchers identified a polyamine entrance binding pocket located in the endo-/lysosomal luminal leaflet with an outward-opening conformation in the E2P structure of hATP13A2. MD simulations confirmed that this pocket was where polyamines were initially captured.
Polyamine translocation. The next step of the conformational cycle is the transition from the E2P state to the E2-Pi state, which involves dephosphorylation of aspartic acid and substrate movement along the transport pathway. They detected substrate binding at multiple potential binding sites along the pathway in cryo-EM structures. By using coarse-grained MD simulations and cross-linking mass spectrometry, they determined that the inward-opening cavity was the second substrate binding site of hATP13A2. This allowed them to capture the key intermediate state during polyamine translocation for the first time.
Polyamine release. After the release of phosphate ions, hATP13A2 reaches the E2 state before releasing the substrate. In the cryo-EM density maps, the researchers observed a large amount of relatively dynamic lipid analogs near the substrate release pocket. To further investigate this observation, they studied the interactions between lipids and polyamines and found that these lipids were likely to be strongly negatively-charged phospholipids, especially PIP2. Finally, through all-atom MD simulations, they located the third and final polyamine binding site, which was highly hydrated. Thus, this revealed a complete polyamine transport pathway.
In summary, the researchers reported the structures of a human polyamine transporter in a nearly complete conformational cycle and proposed a substrate transport mechanism for polyamines. These findings may provide insights for rational drug design against hATP13A2.