Study of Kindlins reveal novel pathways to cancer treatment
A new study has investigated the influence of Kindlins– adapter proteins that exist inside cells of vertebrates, in various cancers. Since this protein is central to many signaling pathways, targeting it could lead to new cancer treatments that address multiple aspects of the disease at once.
Kindlins are adapter proteins that exist inside the cells attached to the cell membranes of almost all types of cells in vertebrates. They transfer extracellular mechanical cues to biochemical signals inside the cells and play a pivotal role in conveying extracellular signals by physically interacting with structural proteins, receptors and transcription factors, triggering a cascade of chemical signals within the cell.
Structural disruptions in these proteins can have a global impact on mechano chemical signaling, leading to disruptions in the state of balance among all the body systems needed for the body to survive and function correctly. This balanced state of the body is called homeostasis.
Kindlins may undergo mutations under the influence of innumerable chemical and physical carcinogens like nicotine, ultraviolet rays and many more. Mutated Kindlin can potentially disrupt global mechanical homeostasis within cells. Therefore, understanding the consequences of genetic alterations in Kindlins holds the key to unraveling the intricate mechanisms leading to the growth of cancer cells.
A team from S. N. Bose National Centre for Basic Sciences in Kolkata, an autonomous institute of the Department of Science and Technology (DST) collected data of 10,000 patients with 33 cancer types from The Cancer Genome Atlas, to understand the role of Kindlins in turning normal cells into cancerous ones.
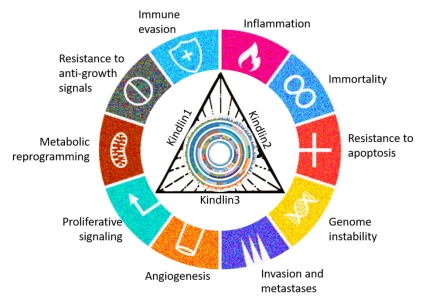
The researchers led by Debojyoti Chowdhury working under the guidance of Prof. Shubhasis Haldar found that Kindlin 1 (belonging to Kindlin family) regulates the immune microenvironment in breast cancer and that cancer-specific metabolic regulation, such asTCA cycle and glycolysis, is governed by Kindlin 2.
Kindlin family of proteins contains three members: Kindlin 1, 2, 3 with distinct amino acid sequences and tissue distribution. “Hippo signaling is a kind of signal in cancer cells that tells the cell to migrate and invade other tissues. Kindlin 2 can also regulate HIPPO signaling,” explained Debojyoti Chowdhury.
The researchers employed structural and functional genomics tools on this data to investigate the influence of Kindlin family proteins on mechano chemical signaling in various cancers. The results highlighted the role of Kindlins in processes related to tumor progression, metastasis and epithelial-mesenchymal transition (EMT). In EMT, cells shift from being more like tightly packed, organized epithelial cells (like those lining our skin) to becoming more free-moving and flexible mesenchymal cells (like those in our muscles). This process happens when cancer cells spread to different parts of the body.
The study strongly suggests that Kindlins participate in essential mechano sensitive pathways. This study also suggests a potential link between Kindlin dysfunction and adverse survival outcomes.
This structural genomics approach establishes associations with clinical parameters, providing evidence for the potential mechanochemical importance of Kindlins across diverse cancer stages and subtypes. “By studying all Kindlin family members collectively, we can gain a comprehensive understanding of their potential complementary and synergistic roles in cancer biology”, says Debojyoti. “This includes examining the interaction of different Kindlin proteins with each other or with other cellular components to influence cancer cell behavior, tumor progression, and response to therapy”.
“The data related to Kindlin family alternations and mutational and stability analyses presented in our work strongly coincide with those of previous experimental studies. We found that Kindlin 2 expression is elevated in breast cancer, and it activates epithelial-mesenchymal transition (EMT)” asserts Chowdhury. Similar results had been obtained in earlier experiments too.
The study published in the journal Communications Biology has helped in deciphering the intricate interplay between tumors and their micro-environment. It has brought out the potentiality of Kindlins as promising targets for innovative mechano-modulatory cancer therapeutics, offering context-dependent avenues for intervention and treatment strategies.
Chemoresistance and tumor relapse are two major challenges faced by oncologists. The present study will serve as a beacon for developing future therapeutic strategies, targeting the roles of Kindlins in cancer treatment. This will open a new strategy in the 4000 years old war against cancer.