Study Shows Triple Combination Therapy Brings Lasting Improvement In Cystic Fibrosis
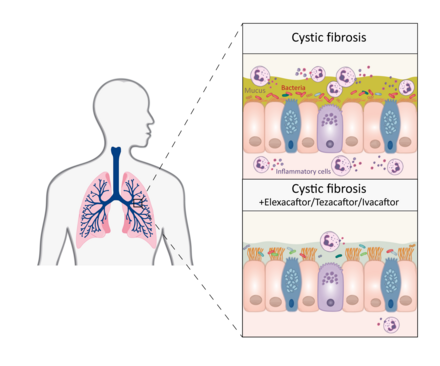
The mucus in the airways is not as sticky, inflammation in the lungs significantly reduced: Triple combination therapy can achieve these positive, lasting effects in patients with cystic fibrosis (CF). Researchers from Charité – Universitätsmedizin Berlin and the Max Delbrück Center have just recently published their findings in the European Respiratory Journal.* According to their research, this form of medication improves the symptoms of CF in many patients.
Two years ago, a research group headed by Charité showed that combination therapy involving three drugs – elexacaftor, tezacaftor, and ivacaftor – is effective in a large portion of patients with cystic fibrosis, a hereditary disease, meaning that the treatment noticeably improves both lung function and quality of life. Now, the team headed by Prof. Marcus Mall, who has been the lead researcher in both studies, has investigated for the first time whether this form of treatment is also helpful in the long term, meaning over a period of 12 months or more. To examine this, the researchers took a closer look at the sputum, the secretions from patients’ respiratory tracts. “In patients with cystic fibrosis, the mucus in the airways is very sticky because it doesn’t contain enough water and the mucins, the molecules that form mucus, adhere too much due to their chemical properties. This results in thick, sticky mucus, which clogs the airways, making it harder for patients to breathe and leading to chronic bacterial infection and inflammation of the lungs,” explains Mall, Director of the Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine and the Christiane Herzog Cystic Fibrosis Center at Charité.
In the current study, the researchers show that a combination of elexacaftor, tezacaftor, and ivacaftor results in less viscous respiratory secretions and decreasing inflammation and bacterial infection in the lungs of cystic fibrosis patients. “What’s more, the effects lasted over the entire one-year study period. This is really important because previous medications caused a rebound in the bacterial load in the airways,” explains Dr. Simon Gräber, who also works in the Department of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine at Charité and was one of the co-leaders of the study. 79 adolescents and adults with cystic fibrosis and chronic lung disease participated in the trial.
A major step in treating cystic fibrosis, further research important
“This is a major step forward in treating cystic fibrosis,” Mall says. “At the same time, it would be premature to say that patients have been normalized, let alone cured. Chronic lung changes arising over many years of living with the disease cannot be reversed, unfortunately.” This means patients with advanced lung disease will still need to rely on established treatments involving inhaling mucus-thinning medications, taking antibiotics, and physical therapy.
“We plan to forge ahead with our research on how to make treatments that address cystic fibrosis via the molecular defects that cause the disease – like the triple medication combination studied here – even more effective. This includes starting treatment in early childhood with the goal of preventing chronic lung changes wherever possible,” Mall notes. “Aside from that, this therapy is not available to about ten percent of our patients right now due to their genetic conditions,” Gräber adds. “That’s why we are also hard at work on research involving new molecular treatments so we can treat all people with cystic fibrosis effectively.”
The researchers are also working to advance their understanding of mucus defects in cystic fibrosis and develop new mucolytics, drugs that thin and loosen the mucus. This research could also benefit patients with common chronic inflammatory lung diseases such as asthma and COPD.