UC San Diego Unveils New ‘Atlas’ Offering Unprecedented Insights into Early Embryo Development and Gene Function
Although the Human Genome Project announced the completed sequencing of 20,000 human genes more than 20 years ago, scientists are still working to grasp how fully formed beings emerge from basic genetic instructions.
Biomedical efforts to learn how disorders can take hold in the earliest stages of development would benefit from knowing specifically how complex organisms arise from a single fertilized cell. Researchers from the University of California San Diego have captured a new understanding of how embryonic development unfolds through the lens of a simple model organism.
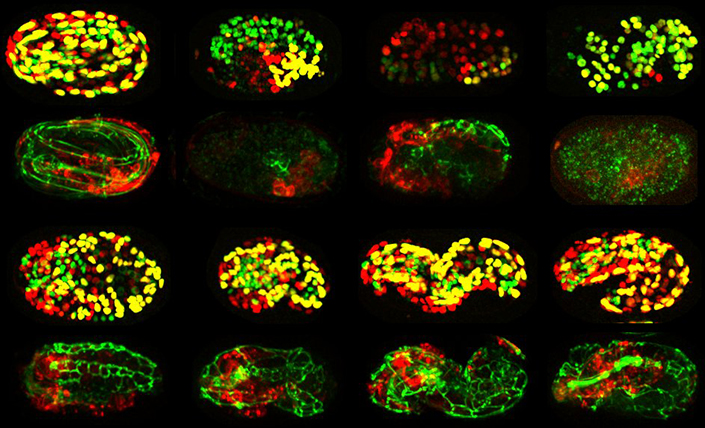
This gallery depicts a collection of embryos after genes were blocked one at a time. The distinct outcomes (or characteristics observed) for each embryo reflects the specific functions of the genes tested.
The comprehensive report led by School of Biological Sciences scientist Rebecca Green and Professor Karen Oegema provides a play-by-play of how genes function during embryonic development in Caenorhabditis elegans (C. elegans), a millimeter-long roundworm known to biologists as “the worm.” Despite its tiny size, C. elegans has been a workhorse for scientists because so much of its biology, including early developmental stages, resembles that of higher organisms, including humans. The research, which forges a decade’s worth of work by a collaborative multidisciplinary team into a “genetic atlas,” is published in the journal Cell.
“By characterizing many of these poorly understood genes in a simple model organism, we can learn about what they are doing in more complex systems like humans,” said Green, a bioinformatics scientist and first author of the paper. “While the work is done using C. elegans, the majority of genes analyzed are present in humans and mutations in many of them are associated with human developmental disorders.”
The researchers developed an automated system for profiling the function of genes required for embryogenesis, the process by which a fertilized egg, which starts as a single cell, develops into an organism with different tissues, such as skin, digestive tract, neurons and muscles. They used time-lapse 4-D imaging to methodically track the function of each gene throughout all embryonic stages, including when cell identity is determined and when the tissues in the organism take shape. The researchers monitored this process using an approach known as “computer vision” to track specific aspects of development, including the number of cells in each tissue. They also tracked the mass, position and shape of the tissues within the developing organism.
Biological Sciences researchers developed an automated system for profiling the function of genes required for embryogenesis, the process by which a fertilized egg, which starts as a single cell, develops into an organism with different tissues, such as skin, digestive tract, neurons and muscles.
To fully understand the function of nearly 500 genes that are important in embryonic development, they blocked the function of each gene, one at a time. This allowed the researchers to group genes into common clusters that revealed the role of each gene through “guilt by association.” Green likens the process to automated facial recognition, in which images with features that appear similar are grouped together. By using this meticulous process to analyze a collection of nearly 7,000 4-D embryogenesis movies, the team was able to create “fingerprints” for individual genes, such as those required for cells to become muscle or skin. This helped them understand the physiological roles that the genes play in embryogenesis, such as controlling the formation of tissues like the intestine or nervous system.
“We show that our approach correctly classifies the functions of previously characterized genes, identifies functions for poorly characterized genes and describes new gene and pathway relationships,” said Oegema, a faculty member in the Department of Cell and Developmental Biology and the paper’s senior author. “A lot of genes that we thought served mundane functions were found to have important roles that were underappreciated.”
In conjunction with the Cell paper, the abundance of data from the research led to the launch of a new online resource that houses all of the information. PhenoBank now offers a portal to the genetic atlas developed during the research.
“The approach yielded surprising insights into how metabolic pathways are specialized during embryogenesis and revealed interesting new connections between different molecular machines involved in gene regulation,” said Professor Arshad Desai, a paper coauthor.
Beyond the 500 genes covered in the Cell study, the researchers are now working to finish the entire set of 2,000 C. elegans genes that have been implicated in embryogenesis.
“A lot of genes that we thought served mundane functions were found to have important roles that were underappreciated.”— Professor Karen Oegema
“The broad interest lies in the approach developed to tackle arguably the most challenging problem in biology: how a single cell with a genome that contains approximately 20,000 genes (similar to the number of genes in humans) is able to build an entire organism,” he said.
Authors of the paper included: Rebecca Green, Renat Khaliullin, Zhiling Zhao, Stacy Ochoa, Jeffrey Hendel, Tiffany-Lynn Chow, HongKee Moon, Ronald Biggs, Arshad Desai and Karen Oegema. The researchers also thank Tony Hyman and the Scientific Computing group at Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) for facilitating the PhenoBank build.
The research was supported by the National Institutes of Health (GM147265) and the Ludwig Institute for Cancer Research.