USTC Achieves Long-Lasting CO2 Conversion in Proton-Exchange Membrane System
Professor YAO Tao’s team from the National Synchrotron Radiation Laboratory of University of Science and Technology of China (USTC), Professor XIA Baoyu’s team from Huazhong University of Science and Technology (HUST), and Dr. Ziyun Wang from the University of Auckland in New Zealand, developed a proton-exchange membrane (PEM) system that cut down carbon dioxide (CO2) to formic acid at a catalyst from waste lead-acid batteries.This study was published in Nature.
Developing various carbon-neutral technologies is crucial for addressing energy and environmental challenges. The CO2 reduction reaction (CO2RR) in a strong acid operated under a PEM system holds promise for scalable carbon conversion due to its ability to produce high-value chemicals and fuels with high current and long-term stability.
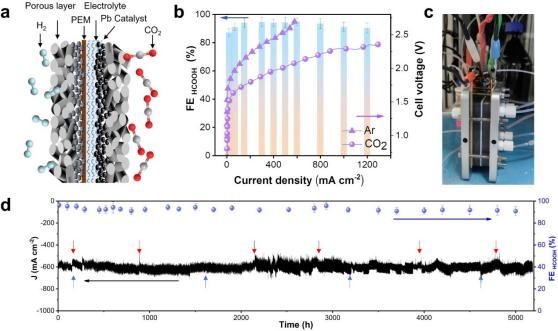
Researchers have achieved high CO2RR activity with cathode recycled Pb (r-Pb) over a wide pH range, producing formic acid with a Faradaic efficiency exceeding 93% under 2.2 V voltage and continuous operation for 5200 hours at a current density of 600 mA cm-2.
The activity and stability of cathode recycled Pb membrane electrode for CO2RR (Image by USTC)
To elucidate the true active structure of r-Pb catalysts in CO2RR, they developed and developed a self-designed membrane electrode CO2RR in situ device suitable for X-ray absorption spectroscopy. Offline and in situ characterizations were conducted at BL12B X-ray magnetic circular dichroism (XMCD) at National Synchrotron Radiation Laboratory of USTC and beamline 1W1B (XAFS) at Beijing Synchrotron Radiation Facility.
In situ X-ray absorption spectroscopy revealed a dynamic structural evolution of r-Pb from Pb/PbSO4 to Pb/PbCO3 under the reduction potential of CO2RR. The coexistence of metallic Pb and PbCO3 at the reduction potential is crucial for the high selectivity and activity of formic acid production.
Further in situ infrared spectroscopy studies of CO2RR were conducted based on the Hefei Light Source in situ infrared spectroscopy technology (BL01B). It was found that gaseous CO2 on the surface of PbCO3 undergoes surface activation before entering the PbCO3 lattice where it is converted into HCOOH products.
Combining with density functional theory calculations, the researchers unveiled the dynamic phase transition-induced lattice carbon activation and CO2 conversion mechanism of r-Pb catalysts.
This study signifies a step forward in understanding and advancing PEM CO2 conversion technology