USTC Develops Catalyst Synthesis Strategy to Optimize Hydrogen Production from Water Electrolysis
A team, led by Academician YU Shuhong from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), delivered a general strategy for the synthesis of ten single-atom doped CoSe2-DETA (DETA = diethylenetriamine) nanobelts. Researchers adjusted the doped single-atom ions and modulated the electronic structure to optimize the hydrogen evolution reaction (HER) activity of the products. This research was published in Nature Communications.
Hydrogen, as a renewable and clean energy, has the potential to replace fossil fuels, and producing “green hydrogen” through HER could be a wise way to embrace a hydrogen-supporting society. Due to the unique electronic structure of the precious metal platinum (Pt), Pt and Pt-based materials are currently the only commercially available HER electrocatalysts. However, their high price and scarcity prevent large-scale commercial applications. Therefore, it is urgent to design low-cost HER electrocatalysts with high catalytic activity and stability.
Optimizing the electronic structure of catalysts can improve the intrinsic properties of the material. Among them, ion doping is an effective method to optimize HER performance by introducing heteroatoms into the material lattice. This strategy changes the local coordination and modulates the electronic structure, which improves catalytic performance. Although previously reported ion-doped electrocatalytic materials exhibit impressive HER performance due to the introduction of isolated metal atoms, their lack of a general strategy leads to insufficient modulation of the microstructure of catalysts, hindering the investigation of the relationship between their properties and structures. Thus, it remains challenging to develop efficient and stable ion-doped HER catalysts with clear structure-property relationships.
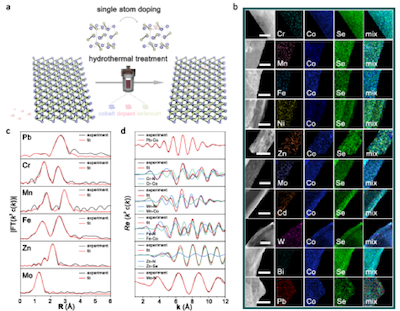
Cobalt diselenides (CoSe2) have an electronic structure similar to Pt, which is often studied as an alternative to Pt for catalyzing HER reactions, but their activity and stability are still inferior to those of Pt and Pt-based materials. Therefore, a general synthetic methodology for such catalysts with high-efficiency HER performance remains to be explored. In this work, researchers developed a general single-atom doping strategy for the preparation of ten single-atom doping products by replacing Co with different cations. Assisted by different doping atomic systems, the local coordination of the products can be modulated to realize the controllable adjustment of electronic structures and HER behaviors over a wide range.
The experimental results showed that the catalytic activity of the optimal doped product is similar to that of commercial Pt/C (40 wt%). The overpotential required to reach a current density of 10 mA/cm2 is only 74 mV, with a Tafel slope of 42 mV/dec calculated from the polarization curve. In addition, accelerated voltammograms (CVs) cycling tests proved the long-term stability of the catalysts. The activity of the catalysts remains almost constant over 1000 CVs cycles, and the product is able to operate stably under a constant current density of 10 mA/cm2 for 20 hours. The synchrotron spectroscopy data showed that the coordination environment of the Co atoms (ratio of Co-N bonds to Co-Se bonds) in the product varies with different dopant atoms. Besides, a volcano-shaped relationship between the coordination environment and the HER activity of the product was summarized, demonstrating the structure-property relationship of doped CoSe2.
This work provides a novel method for the design and synthesis of high-efficiency catalysts, and the optimal products obtained are expected to substitute the commercial Pt/C catalysts and become ideal electrode materials.