USTC New Strategy for Cathode Materials in Li-Cl2 Battery
A team led by Prof. CHEN Wei, Prof. JIANG Hailong and Prof. LI Zhenyu from the University of Science and Technology of China (USTC) adopted NH2-functionalized metal-organic frameworks (MOFs) in lithium-chlorine (Li-Cl2) batteries to achieve high specific capacities, cycle stability and superior low-temperature performance. Their work was published in Joule on March 15th.
Traditional lithium-thionyl chloride (Li-SOCl2) batteries are widely used for their high energy density and other advantages, but alternatives are still needed since Li-SOCl2 batteries are not rechargeable. Rechargeable Li-Cl2 battery was first invented in 2021, with a high specific capacity of 1200 mAh/g and a high output voltage of ~3.6 V.
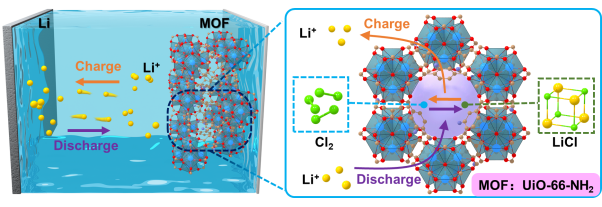
However, the following problems stand in the way of the practical application of Li-Cl2 battery. Firstly, the Cl2 reaction is limited by the weak physical adsorption of porous carbon to Cl2 molecules. Secondly, the excessive LiCl generation into the carbon pores blocks the channels for Li+ transportation and Cl2 diffusion, hindering further electrochemical reactions. Furthermore, the shuttle effect of unbonded Cl2 leads to battery capacity decay, especially at high output capacities. Therefore, the cathode materials with highly porous structures are vital to realize high-performance Li-Cl2 batteries.
To overcome the above difficulties, the team proposed that MOFs with Lewis basic functional groups should be applied to improve the cathode Cl2/LiCl reactions. Guided by theoretical predictions, MOFs with -NH2 functional groups were screened out using first-principles calculation and applied in Li-Cl2 batteries. Cryo-TEM and low-dose high-resolution TEM showed that UiO-66-NH2 maintains a very stable structure during cycling, and XPS verified -NH2 groups’ strong affinity to Cl2 and LiCl, thus enhancing its redox reaction kinetics. Li-Cl2@MOF batteries designed by the team reached a maximum discharge specific capacity of 2000 mAh/g and are stable for more than 500 cycles at a specific capacity of 1000 mAh/g. Meanwhile, the batteries performed stable function under the temperature of -40 °C.
This work presented an innovative design of cathode materials in Li-Cl2 batteries, paving the way for future application of rechargeable Li-Cl2 batteries.