CeNS develops low-cost catalyst for hydrogen generation from water
New Delhi: Scientists from The Centre for Nano and Soft Matter Sciences (CeNS), an autonomous institute of the Department of Science and Technology (DST), have found out a low cost and efficient way to generate hydrogen from water using Molybdenum dioxide as a catalyst.
The scientists have shown that Molybdenum dioxide (MoO2) nanomaterials annealed in hydrogen atmosphere can act as efficient catalysts to reduce the energy input to bring about water splitting with great efficiency. Electrolytic splitting of water is a promising method to generate hydrogen but requires energy input that can be brought down in the presence of a catalyst.
Molybdenum dioxide has the potential to replace the currently employed catalyst Pt, which is expensive and has limited resources. MoO2 is a conducting metal oxide that is one of the low-cost catalysts with good efficiency and stability for hydrogen evolution.
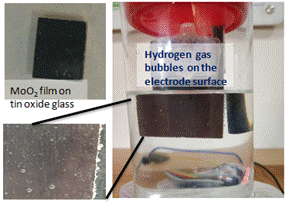
The researchers were able to grow MoO2 directly on to tin oxide substrates for direct use as a catalyst in electrochemical cells, avoiding the need for any further electrode fabrication process. It can also be obtained as powder in high yield from cheaper precursors in an aqueous medium. Their research has been published in Chemistry- a European scientific journal.
Dr. Neena S John and co-workers from CeNS have been able to grow metallic MoO2 nanostructures on tin oxide glass and have shown that the voltage required to obtain high current density (or higher amount of hydrogen) are close to that of Platinum in acidic medium. The catalyst can be easily synthesized in the form of powder as well, with high yield from cheaper reagents such as ammonium molybdate and citric acid in water.
Mr. Alex C., a research scholar, working on this material stresses that ‘this metal oxide nanomaterial is a cheaper alternative to the precious noble metal catalysts such as Platinum, presently employed in industry for water electrolysis.’ The catalyst is highly stable for a longer duration of reaction with sustained hydrogen evolution from water. About 80 % efficient conversion of electrical energy into hydrogen has been achieved using this catalyst.
Hydrogen is considered as the future of clean and sustainable energy as it can be generated from water and produces water on energy generation without any carbon footprint. Hydrogen can be directly used as a fuel similar to natural gas or as input for fuel cells to generate electricity. It is the future energy for a clean environment and an alternative to fossil fuels, underlining the necessity of low-cost catalysts for its production.