Leiden University: €10.6 Million Allocated for Innovative Toolboxes to Combat Brain Cancer
Researchers at the Universities of Amsterdam (Uva) and Leiden together with the Netherlands Cancer Institute and Oncode Institute have received a €10,6 million ERC Synergy Grant to develop innovative therapeutic approaches to target glioblastoma. This is a deadly primary brain tumour for which no curing therapy is yet available. At the heart of the proposed method are enzyme-mimicking molecular catalysts capable of producing different type of anti-cancer drugs within the tumour tissue itself. According to coordinator of the ‘Cat4CanCenter’ Joost Reek (UvA), this new approach could potentially solve many of the difficulties associated with the current treatment of glioblastoma.
Every year in the Netherlands some 500 to 700 people are diagnosed with glioblastoma. Neither surgery, radiotherapy, nor chemotherapy have yet provided a cure for this form of brain cancer. The challenge faced by surgical and radiotherapy teams is that the tumours are not sharply defined and invade deep into the surrounding brain tissue. The challenge for drug-based therapy is that glioblastoma cells are very effective in developing resistance. Even novel immunotherapy approaches – which have proved impressively successful in other solid tumours – have failed in the case of glioblastoma. This is largely due to a highly suppressive tumour microenvironment, where the so-called tumour-associated macrophages effectively ‘hijack’ the local immune response.
A radically new approach to chemotherapy
In search of a solution, the Cat4CanCenter project takes a radically new approach. It combines the efforts of Prof. Joost Reek, an expert in catalysis at the UvA, Prof. Alexander Kros of Leiden University, specialising in drug delivery, and Dr Leila Akkari, a cancer immunologist at the Netherlands Cancer Institute (NKI) and Oncode Institute. Together, they propose a pioneering form of chemotherapy where active, toxic drugs are synthesised in the tumour environment itself. To enable this, first a molecular catalyst is delivered into the tumour tissue using lipid nanoparticles. Next, so-called pro-drug molecules are administered. Finally, the catalyst converts these non-toxic precursors into active drugs that will fight the glioblastoma ‘from within’.
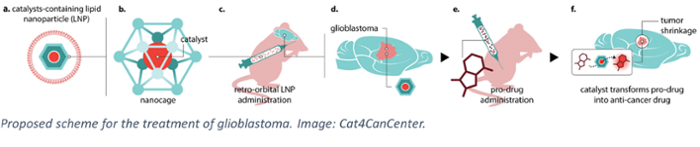
The advantage of this approach is that the tumour can be ‘loaded’ with high levels of drugs without the adverse effects normally associated with drug treatments. Current therapies involve administering high doses in the bloodstream to ensure that enough drugs can cross the blood-brain barrier and arrive at the brain tumour site. However, typically the resulting active dose in the tumour is relatively low, while the high dose in the blood results in concurrent toxicity and off-target side effects. By using non-toxic compounds, the new approach avoids these often severe complications.
Targeted delivery using lipid nanoparticles
Of course, the novel Cat4CanCenter approach also requires the components to cross the blood-brain barrier and to hit specific cell types in the tumour. For the pro-drugs this can be achieved through a dedicated molecular design. For the catalyst, this is ensured by using lipid nanoparticles that can carry compounds from the blood to the brain. This is the expertise of Prof. Kros. ‘The brain is protected from foreign (bio)chemicals by the blood-brain barrier,” he explains. “It has shown to be a formidable barrier, rendering state-of-the-art drug delivery approaches that are successful in other therapies ineffective. In Cat4CanCenter we therefore develop radical new approaches to bring the catalysts inside the tumour by hijacking as yet unexplored existing biological pathways that are already present in the human body.’
Effective metal catalysis
Prof. Reek will use his expertise to design the caged catalysts that will perform the synthesis of the desired drug molecules. He explains that in recent years his team has developed caged catalysts that allow metal-catalyzed synthesis in a biological environment. ‘They now function in an aqueous environment, in the presence of biological molecules and even in living cells. By designing the cage so that is has affinity for the prodrug, we can make the catalyst operate in an enzyme-like fashion, boosting the conversion efficiency at low concentrations.’ He now hopes to unleash this power of metal catalysis for the treatment of cancer, but knows it will be challenging: ‘We need to make sure we can synthesise a whole range of drug molecules to combat all aspects of glioblastoma rapid growth.’
Concurrent remedies
Indeed, the Cat4CanCenter will investigate multiple drugs that together target the combined crucial aspects of the aggressive behaviour of glioblastomas. The effectiveness of these strategies will be studied in detail in near-patients model systems and pre-clinical studies at the Netherlands Cancer Institute directed by Dr Akkari. Her expertise lies in particular in the glioblastoma immune response and the tumour-assisted macrophage biology. ‘Brain tumour research has long suffered from a lack of efficient tools able to bring therapies to this complex cancer site,’ she says, ‘In this project, we will merge a set of expertise never yet combined. It will be challenging, but the outlook has the potential to revolutionise the next generation of cancer therapeutics.’

